Study Notes

Overview
Welcome to your guide on mastering scientific vocabulary and terminology for WJEC GCSE Combined Science (4.5). This isn't just about learning new words; it's about understanding how to use them with precision to unlock higher marks across all your science papers. Examiners are trained to reward candidates who can communicate their scientific understanding clearly and accurately. Using a term like 'pathogen' instead of 'germ', or 'mass' instead of 'weight', demonstrates a deeper level of knowledge and is essential for accessing the top bands in 6-mark Quality of Extended Response (QER) questions. This topic is a cross-cutting theme, meaning it applies to Biology, Chemistry, and Physics, making it one of the most high-impact areas you can focus on for your revision. By mastering the language of science, you are building the foundation for success in every exam question you face.
Key Concepts
Concept 1: Precision Over Colloquialism
In science, words have very specific meanings. A common mistake is using everyday, or colloquial, language instead of the correct scientific term. Examiners cannot award marks for vague or incorrect terms, even if they think they know what you mean. It is your responsibility to be precise.
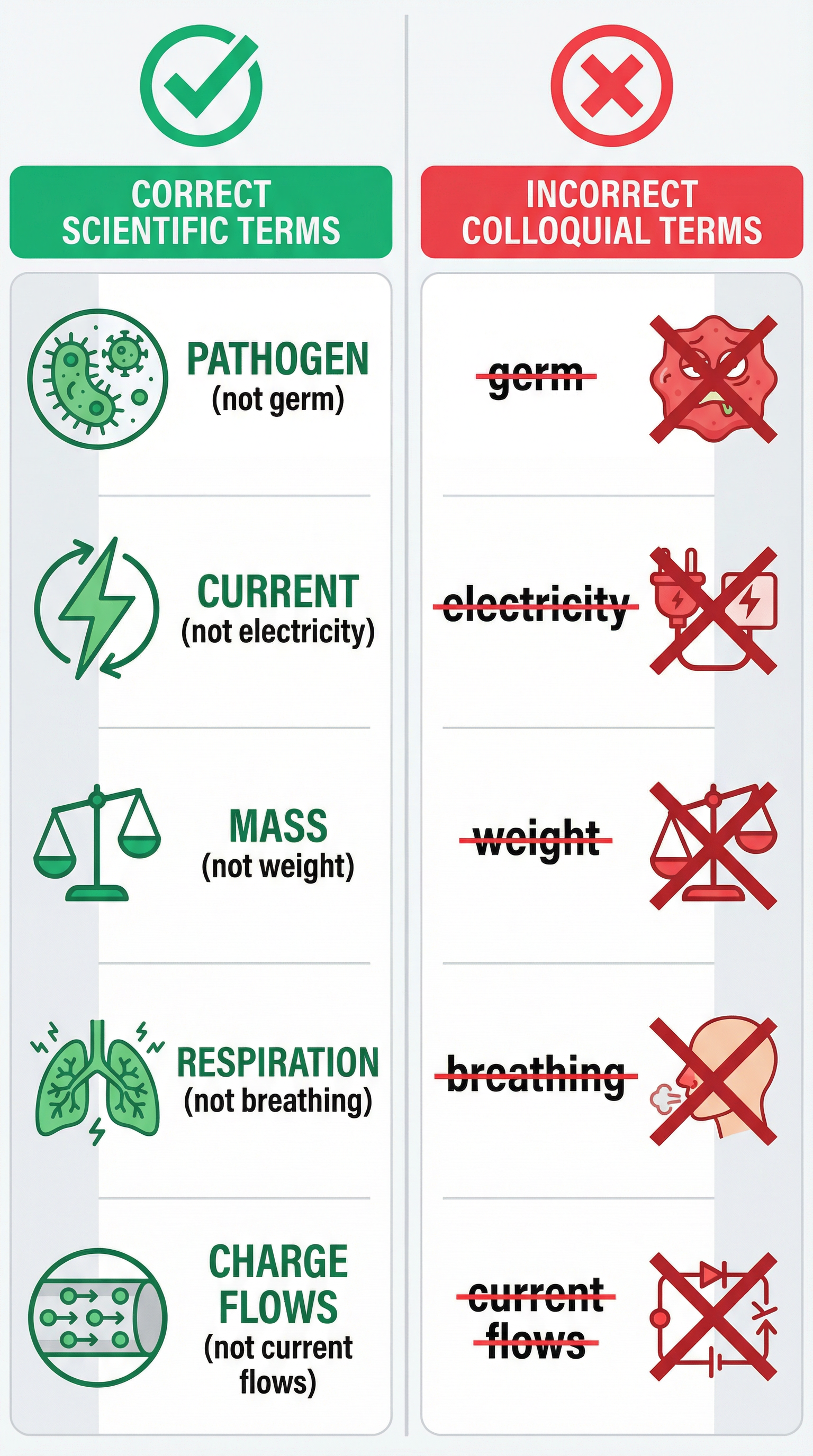
Example: Saying a person has 'caught a germ' is conversational. The correct scientific statement is that they have been 'infected by a pathogen'. A pathogen is a specific term for a microorganism that causes disease, such as a bacterium or virus. 'Germ' is too general and will not be credited.
Concept 2: Distinguishing Between Commonly Confused Pairs
Certain pairs of words are frequently confused by candidates, leading to lost marks. It is vital to learn the exact definitions and contexts for these terms.
- Mass vs. Weight: Mass is the amount of matter in an object, measured in kilograms (kg). It is a constant value. Weight is the force of gravity acting on an object's mass, measured in newtons (N). It can change depending on the gravitational field strength. An object has the same mass on Earth and the Moon, but its weight is much less on the Moon.
- Breathing vs. Respiration: Breathing (or ventilation) is the physical process of moving air into and out of the lungs. Respiration is a chemical reaction that occurs in all living cells to release energy from glucose. Breathing gets the oxygen into the body, but respiration is how that oxygen is used to produce ATP.
- Accuracy vs. Precision: Accuracy is how close a measurement is to the true value. Precision is how close repeated measurements are to each other. You can have precise results that are not accurate if there is a systematic error in your experiment.
Concept 3: The Language of Extended Response Questions (QER)
For 6-mark QER questions, the quality of your written communication is assessed alongside your scientific knowledge. The mark scheme has specific criteria for language. To enter the top mark band (5-6 marks), your answer must be coherent, logical, and use 'relevant scientific terminology with few or no errors'. This means you can have a scientifically sound answer but be limited to 4 marks if your vocabulary is weak.
Strategy: Before writing your answer, jot down 5-6 key terms relevant to the question. As you write, tick them off. This ensures you are embedding the required language into your response.
Concept 4: Understanding Command Words
Every exam question starts with a command word. This word tells you exactly what the examiner expects you to do. Misinterpreting the command word is a very common way to lose marks.
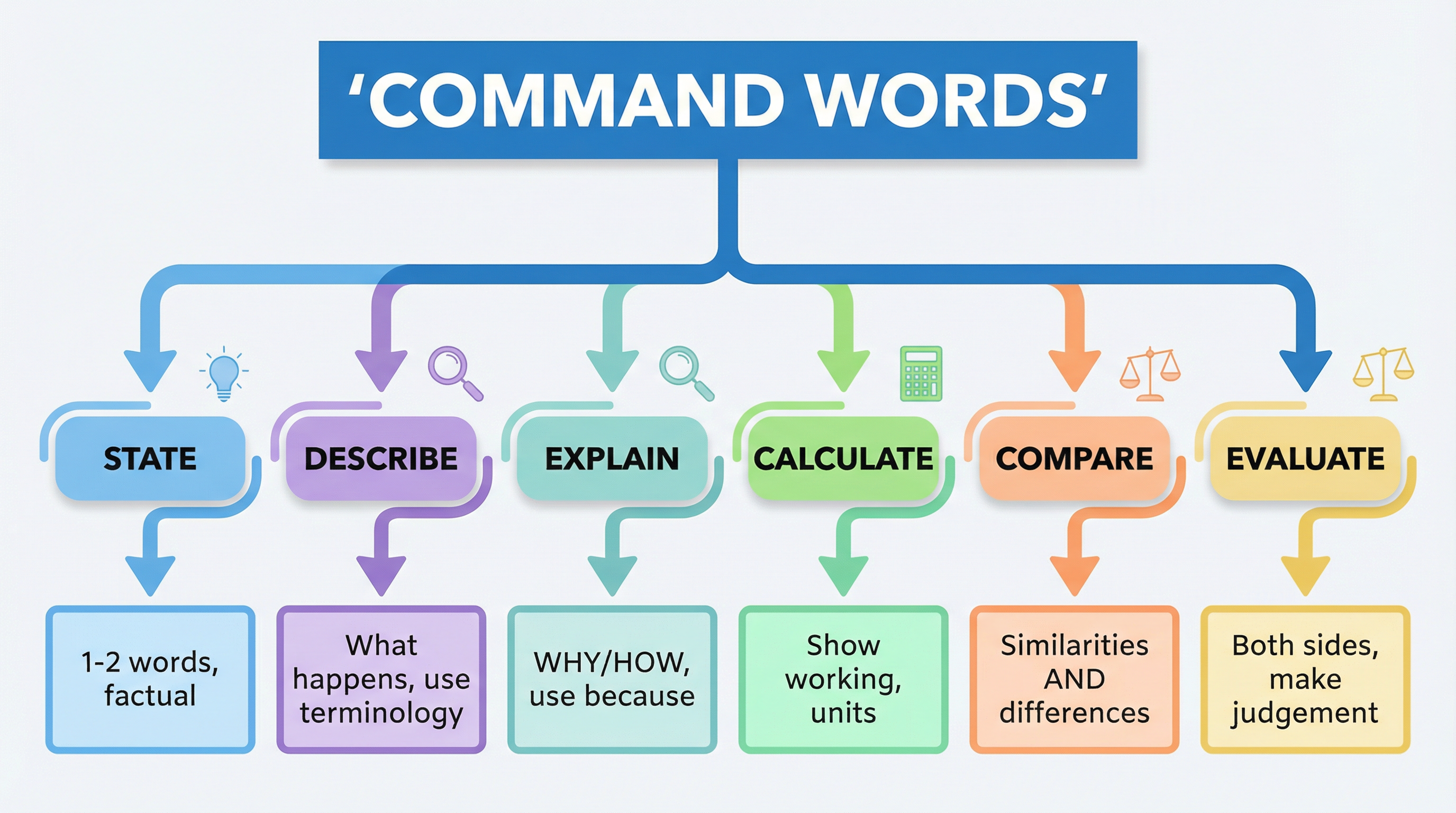
- Describe: State the main features or steps. For example, 'Describe the structure of a cell'.
- Explain: Give reasons why or how something happens. You must use scientific principles. Look to use the word 'because'. For example, 'Explain why enzymes denature at high temperatures'.
- Compare: Identify both similarities and differences between two or more things.
- Evaluate: Use the information provided (and your own knowledge) to make a judgement. You should consider pros and cons or strengths and weaknesses.
Mathematical/Scientific Relationships
While this topic is not formula-heavy, the use of correct terminology is critical when dealing with equations.
- Density Formula: Density = Mass / Volume (ρ = m/V). You must use the term 'mass', not 'weight'. Marks can be lost for stating 'Density = Weight / Volume'.
- Force Equation: Force = Mass x Acceleration (F=ma). Again, 'mass' is the required term.
- Units: Always use the correct SI units. For example, mass in kg, volume in m³, and concentration in mol/dm³. Prefixes like kilo- (k), milli- (m), and micro- (μ) must be used correctly.
Practical Applications
In any required practical write-up, using correct terminology is essential for describing apparatus, methods, and observations.
- Apparatus: Use specific names like 'beaker', 'pipette', 'burette', or 'measuring cylinder' instead of vague terms like 'cup' or 'tube'.
- Observations: Instead of 'the solution fizzed', a better description would be 'effervescence was observed' or 'a gas was produced'.
- Evaluation: When evaluating a method, use terms like 'accuracy', 'precision', 'repeatability', and 'reproducibility' correctly. For example, 'Repeating the measurements and calculating a mean improves the precision of the result'.
