Study Notes
Overview
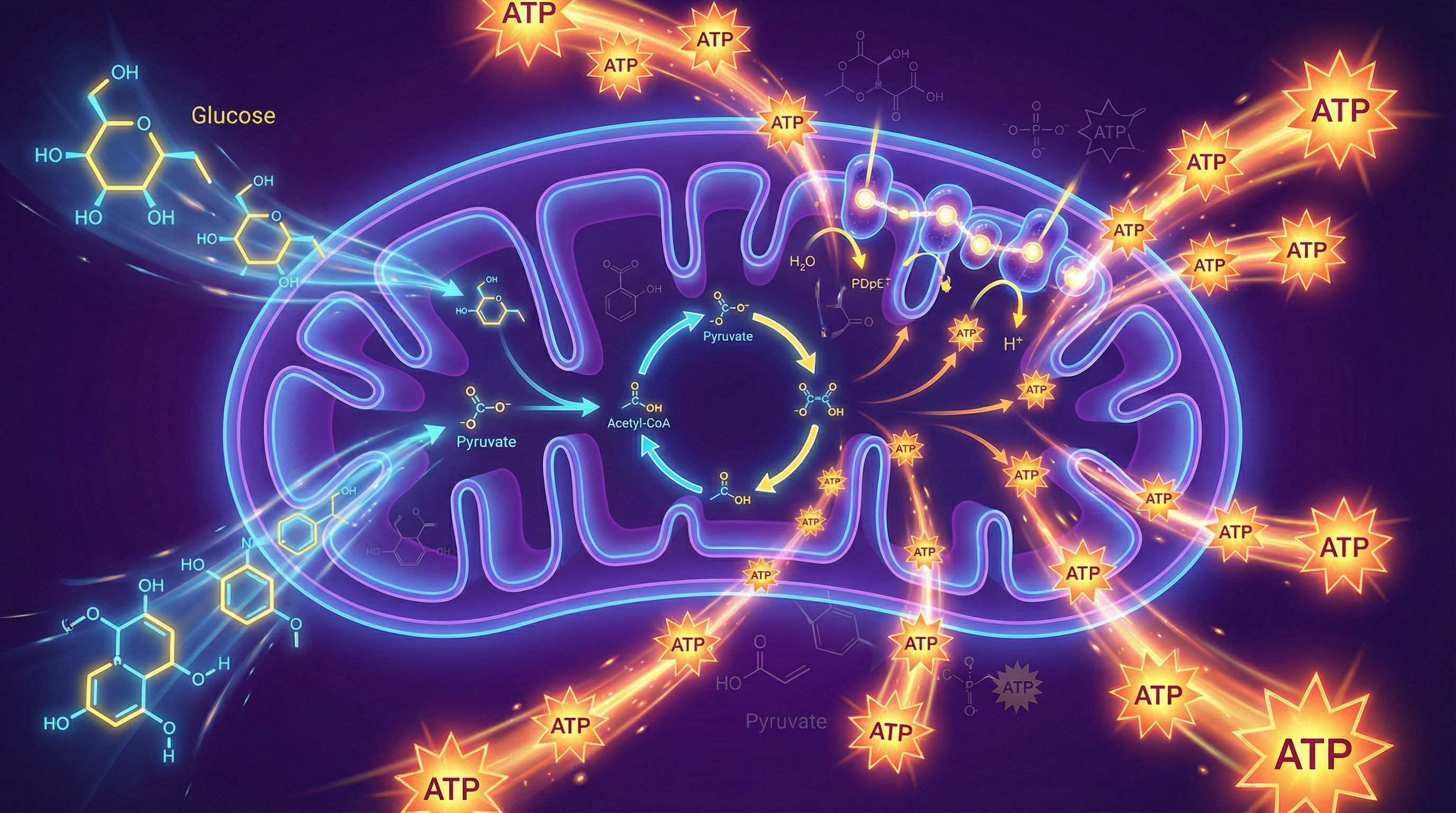
Cellular respiration is the biochemical engine driving life, converting the chemical energy stored in glucose into ATP (adenosine triphosphate), the universal energy currency of the cell. For OCR A-Level Biology (specification 4.3), this topic is a cornerstone of Module 5, demanding a detailed understanding of its four stages, the specific locations within the cell, and the roles of coenzymes and electron carriers. Examiners frequently test this through long-answer questions requiring a sequential explanation of the process, data analysis from respirometer experiments, and synoptic links to topics like photosynthesis and membrane transport. A robust grasp of respiration is not just about memorising pathways; it's about understanding a dynamic, highly regulated process central to metabolism and survival. This guide will equip you with the conceptual understanding and exam technique needed to tackle any question with confidence.
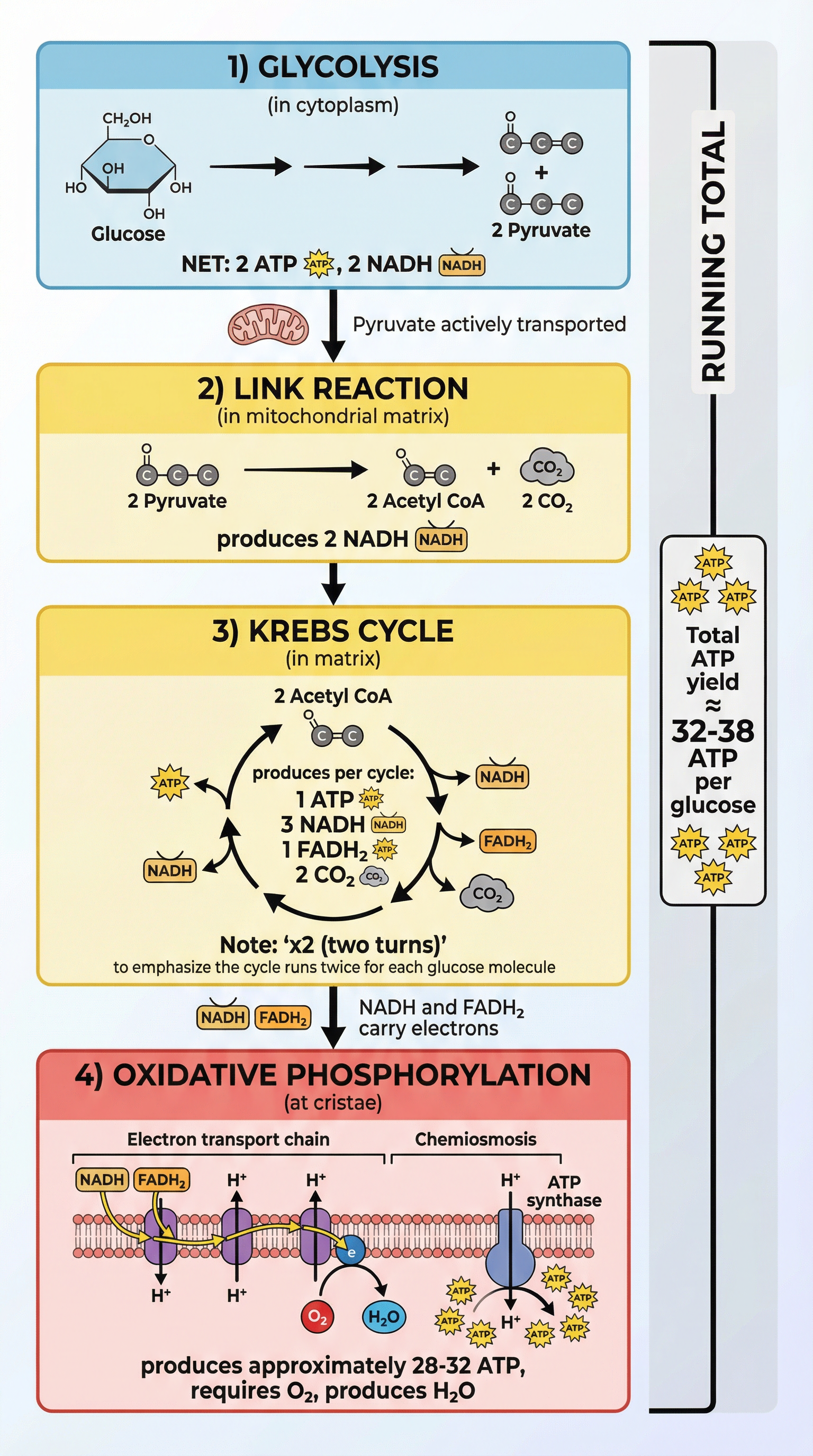
Key Concepts
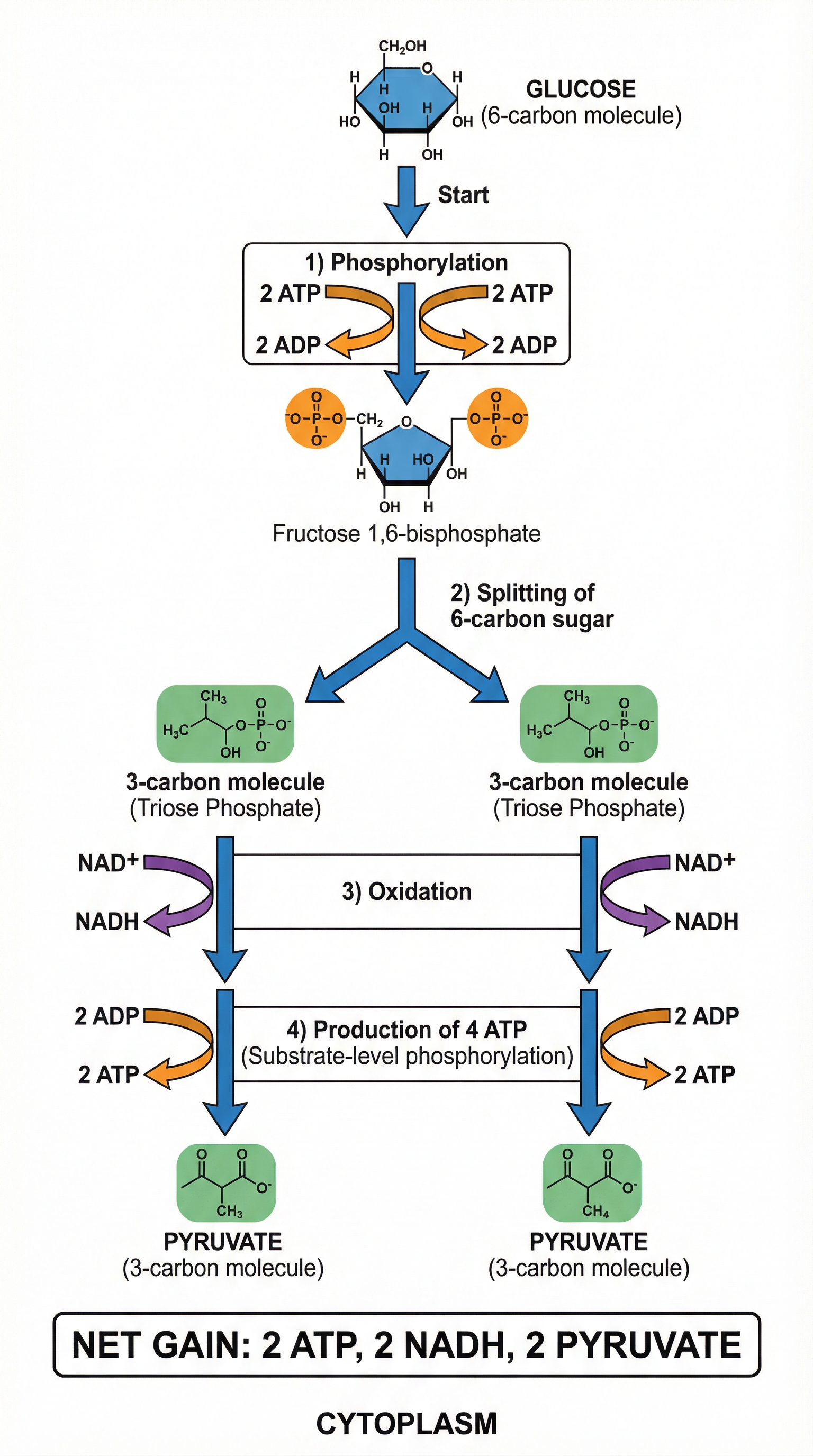
Concept 1: Glycolysis - The Universal First Step
Glycolysis ('sugar splitting') is the initial stage of respiration and occurs in the cytoplasm of the cell. This is a critical detail as it means glycolysis does not require mitochondria and can happen in both aerobic and anaerobic conditions. The process begins with a single molecule of glucose (a 6-carbon sugar) and ends with two molecules of pyruvate (a 3-carbon sugar).
The Process:
- Phosphorylation: The glucose molecule is destabilised by the addition of two phosphate groups, which come from two molecules of ATP. This forms fructose-1,6-bisphosphate. This initial investment of 2 ATP is often referred to as the 'activation energy' of the process.
- Lysis: The unstable 6-carbon sugar splits into two 3-carbon molecules of triose phosphate.
- Oxidation & ATP Formation: Each triose phosphate molecule is then oxidised. Hydrogen is removed (dehydrogenation) and transferred to the coenzyme NAD+, forming reduced NAD (NADH). This is a redox reaction. In the final steps, four molecules of ATP are generated through substrate-level phosphorylation, where a phosphate group is transferred directly from an intermediate compound to ADP.
Net Yield: Because 2 ATP were used to start the process and 4 ATP were made, the net gain is 2 ATP. Additionally, 2 molecules of NADH and 2 molecules of pyruvate are produced per glucose molecule.
Concept 2: The Link Reaction & Krebs Cycle - The Mitochondrial Hub
Following glycolysis, if oxygen is present, the pyruvate molecules are actively transported into the mitochondrial matrix to continue aerobic respiration.
**The Link Reaction:**This reaction connects glycolysis to the Krebs cycle. Each 3-carbon pyruvate molecule undergoes oxidative decarboxylation:
- A carbon atom is removed, forming a CO2 molecule (decarboxylation).
- The remaining 2-carbon acetyl group is oxidised, with the removed hydrogen being transferred to NAD+ to form NADH (dehydrogenation).
- The acetyl group then combines with Coenzyme A to form acetyl CoA.
Since this happens for each of the two pyruvate molecules, the products per glucose are: 2 Acetyl CoA, 2 NADH, and 2 CO2.
**The Krebs Cycle (Citric Acid Cycle):**This is a cyclical series of reactions that also occurs in the mitochondrial matrix.
- The 2-carbon acetyl CoA combines with a 4-carbon molecule (oxaloacetate) to form a 6-carbon molecule (citrate).
- The citrate molecule then undergoes a series of redox reactions, releasing the stored energy. In a single turn of the cycle, the 6-carbon molecule is decarboxylated (twice, releasing 2 CO2) and dehydrogenated, eventually regenerating the 4-carbon oxaloacetate.
- The energy released is captured to produce 1 ATP via substrate-level phosphorylation, 3 molecules of NADH, and 1 molecule of FADH2 (another coenzyme).
Total Yield from Link & Krebs (per glucose): Since the cycle turns twice for each glucose molecule, the total yield is 2 ATP, 8 NADH, 2 FADH2, and 6 CO2.
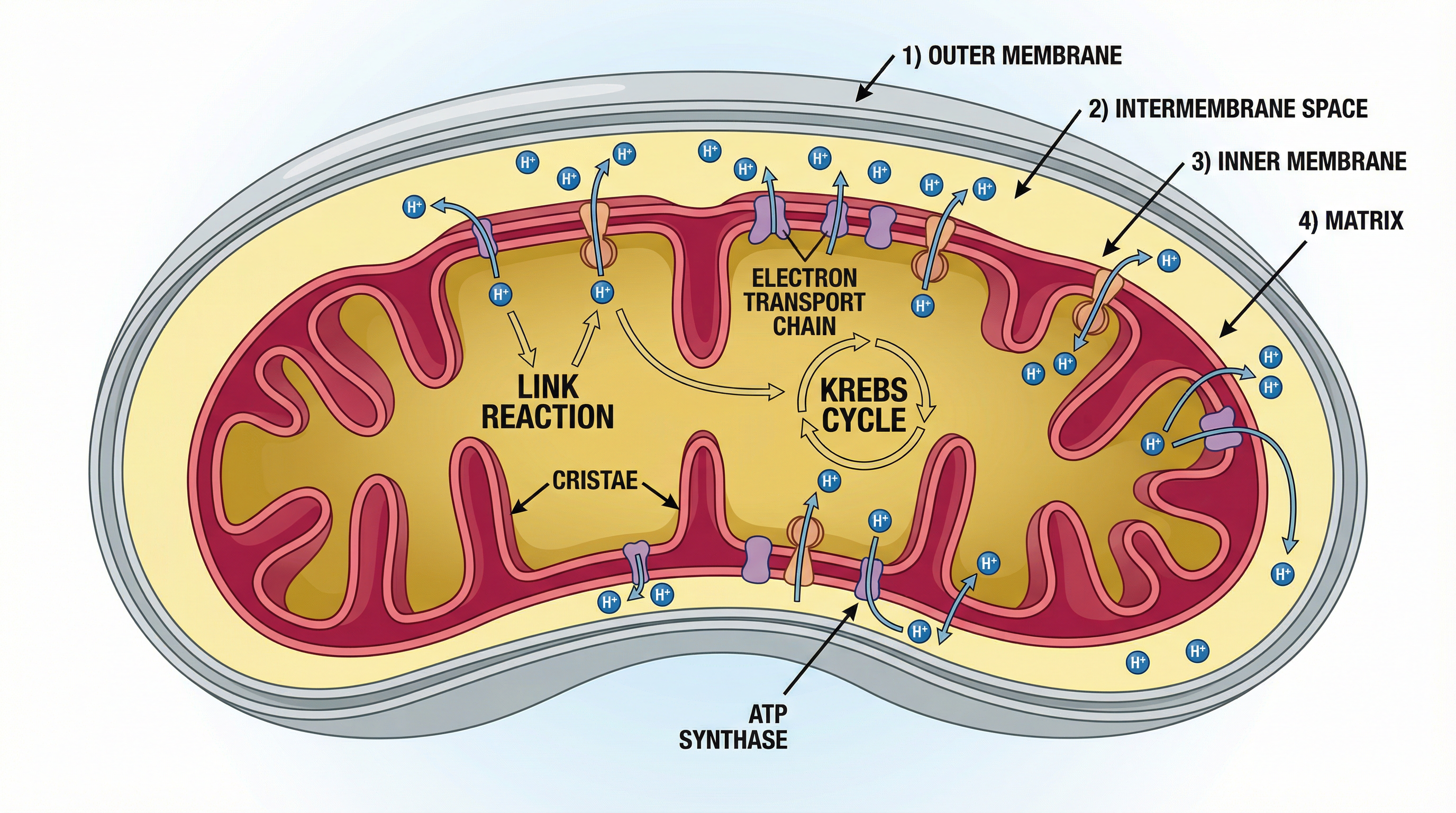
Concept 3: Oxidative Phosphorylation - The ATP Powerhouse
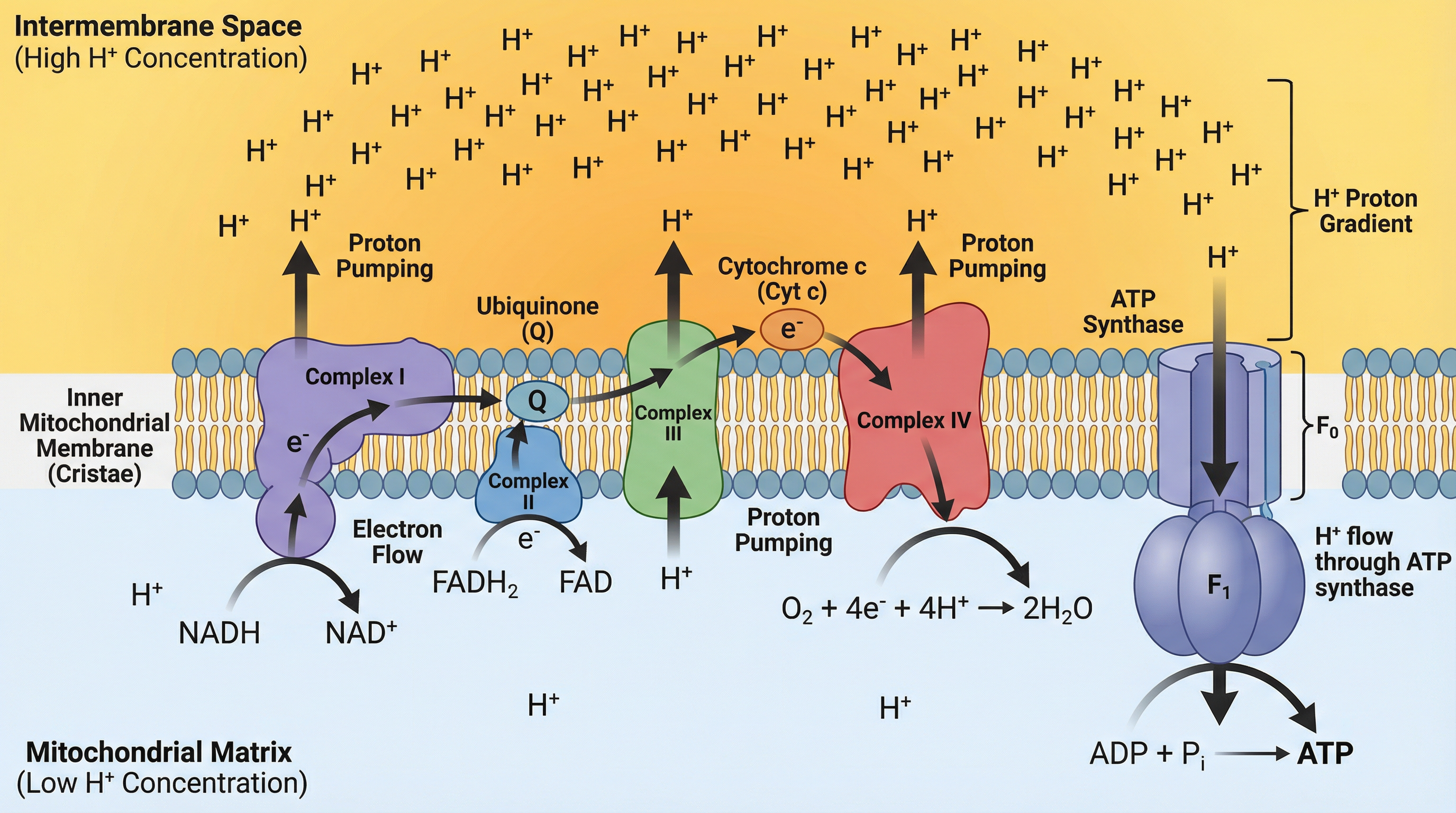
This is the final stage and where the vast majority of ATP is made. It occurs on the inner mitochondrial membrane (cristae) and involves two coupled processes: the Electron Transport Chain and Chemiosmosis.
The Electron Transport Chain (ETC):
- The reduced coenzymes (NADH and FADH2) produced in the earlier stages travel to the cristae and donate their high-energy electrons to the first protein carrier in the chain.
- The electrons are passed down a series of protein carriers (cytochromes) in a cascade of redox reactions, losing energy at each step.
- This released energy is used by the protein carriers to actively pump protons (H+ ions) from the mitochondrial matrix into the intermembrane space.
- This creates a steep electrochemical gradient – a high concentration of protons in the intermembrane space and a low concentration in the matrix. This is often called a proton motive force.
Chemiosmosis:
- The protons cannot simply diffuse back across the impermeable inner membrane. They flow down their concentration gradient through a specific channel protein called ATP synthase.
- The flow of protons through ATP synthase causes it to spin, catalysing the phosphorylation of ADP to ATP. This process is called oxidative phosphorylation because the energy for phosphorylation comes from the oxidation of the coenzymes.
- At the very end of the chain, oxygen acts as the final electron acceptor. It combines with the de-energised electrons and protons from the matrix to form water (O2 + 4e- + 4H+ → 2H2O). This is why oxygen is essential for aerobic respiration.
Mathematical/Scientific Relationships
**1. Respiratory Quotient (RQ)**This is the ratio of CO2 produced to O2 consumed in respiration.
- Formula: RQ = CO2 produced / O2 consumed
- Use: It helps determine the type of respiratory substrate being used.
- Values (Must memorise):
- Carbohydrate (e.g., glucose): RQ = 1.0
- Lipid (e.g., fatty acids): RQ ≈ 0.7
- Protein: RQ ≈ 0.9
- Anaerobic respiration: RQ is undefined (no O2 consumed) or approaches infinity.
2. ATP Yield
- The theoretical maximum yield is 32-38 ATP per glucose. The range exists due to shuttle mechanisms for NADH from glycolysis and proton leakage.
- Rule of thumb for calculations:
- 1 NADH → ~2.5 ATP
- 1 FADH2 → ~1.5 ATP
- Important: Examiners often state that the theoretical yield is rarely achieved in practice. Credit is given for explaining why (e.g., proton leakage across the cristae, ATP used to actively transport pyruvate into the matrix).
Practical Applications
Required Practical: Using a RespirometerA respirometer is used to measure the rate of oxygen consumption by an organism, which provides an indirect measure of its metabolic rate.
- Apparatus: Sealed container with the organism (e.g., woodlice, germinating seeds), a capillary tube with a coloured fluid (manometer), a syringe, and a chemical to absorb CO2.
- Method:
- A known mass of organism is placed in the container.
- Soda lime (or potassium hydroxide) is placed in the container to absorb all CO2 produced by the organism. This is crucial: it ensures that any change in gas volume is due to O2 consumption only.
- The apparatus is sealed and allowed to equilibrate for a set period (e.g., 10 minutes) to allow for pressure changes due to temperature to stabilise.
- The syringe is used to set the manometer fluid to a known level.
- As the organism respires, it consumes O2, reducing the pressure inside the tube and causing the fluid to move towards the organism.
- The distance moved by the fluid over a set time is measured. The volume of O2 consumed can be calculated using the formula for the volume of a cylinder (πr²l), where r is the radius of the capillary tube and l is the distance moved.
- Control: A parallel respirometer is set up with glass beads of the same mass as the organism to control for any changes in atmospheric pressure or temperature.
- Exam Focus: 6-mark questions often ask for a full method. Key marking points include the role of soda lime, the need for equilibration, and the use of a control.
{{asset:respiration_podcast.mp3