Study Notes
Overview
Welcome to the core of animal biology: understanding how millions of cells cooperate to create a functioning organism. This topic, AQA specification 2.1, explores the hierarchy of organisation in animals. We begin with the smallest unit, the specialised cell, and build our way up through tissues and organs to entire organ systems. A mastery of this concept is essential as it provides the foundation for understanding more complex physiological processes later in the course. In this guide, we will focus on two key systems: the digestive system, a chemical processing plant, and the circulatory system, the body's transport network. Expect exam questions to test not just your recall of structures, but your ability to explain how the structure of a component is perfectly adapted for its function. This is a topic where linking ideas is rewarded, so look for connections between enzymes, digestion, and blood circulation.
Key Concepts
Concept 1: The Hierarchy of Organisation
The animal body is organised in a clear hierarchy. This is a concept that examiners expect you to know perfectly.
- Specialised Cells: These are the basic building blocks, adapted for a particular function (e.g., muscle cells for contraction, red blood cells for carrying oxygen).
- Tissues: A tissue is a group of similar cells working together to perform a shared function (e.g., muscular tissue contracts to bring about movement).
- Organs: An organ is a structure made up of a group of different tissues, working together to perform a specific, complex function (e.g., the stomach is made of muscular, glandular, and epithelial tissues).
- Organ Systems: An organ system is a group of organs that work together to perform a major function in the body (e.g., the digestive system breaks down and absorbs food).
Example: A red blood cell is a specialised cell. Together, many red blood cells form part of the blood, a tissue. The blood is circulated by the heart, an organ. The heart is part of the circulatory system, an organ system.
Concept 2: The Digestive System
The digestive system is a classic example of an organ system where multiple organs work in a coordinated sequence to break down large, insoluble food molecules into small, soluble molecules that can be absorbed into the bloodstream.
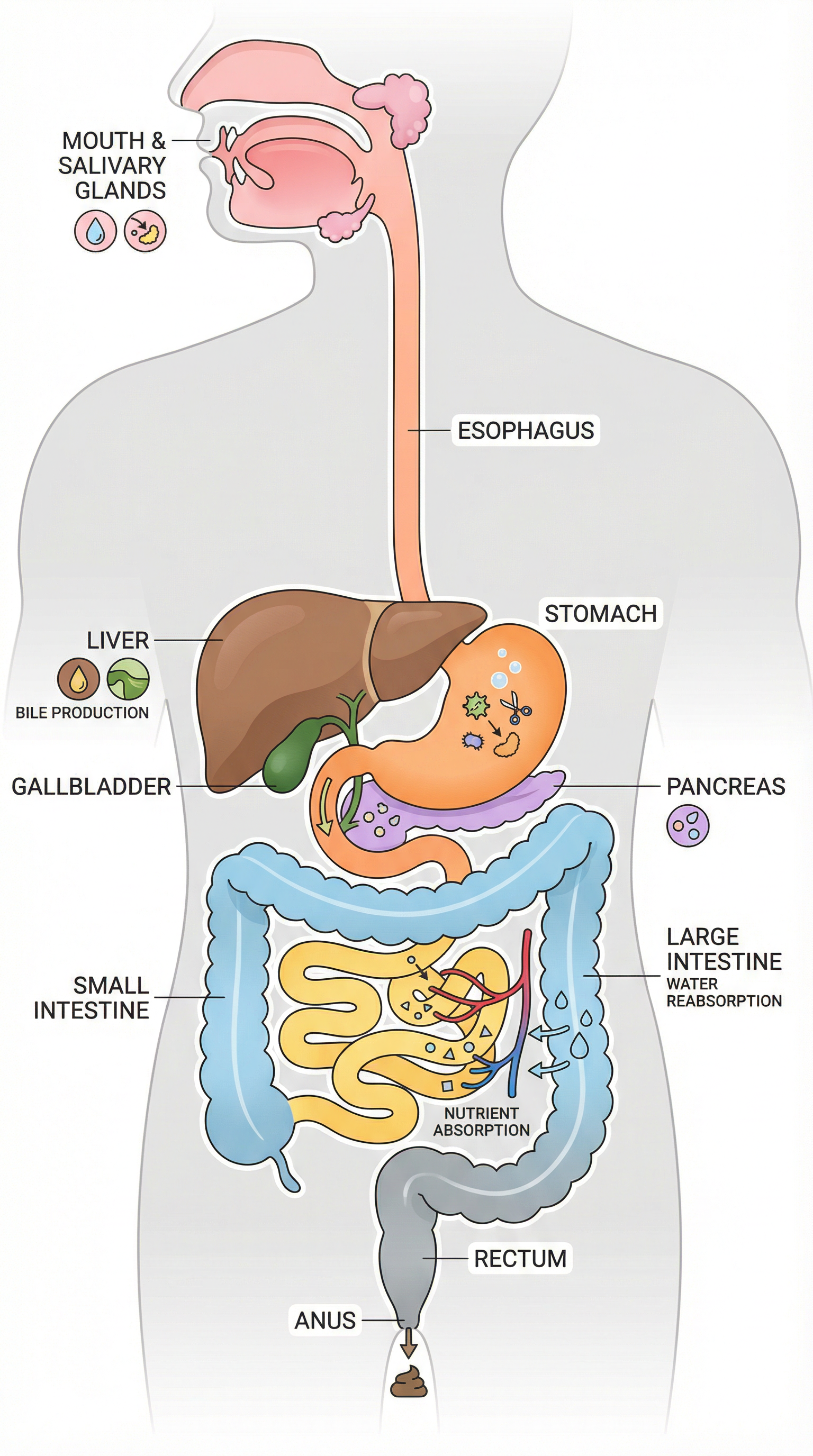
- Mouth & Salivary Glands: Mechanical digestion by teeth; chemical digestion of starch by amylase.
- Stomach: Produces hydrochloric acid (HCl) to kill pathogens and provide the optimum low pH for the protease enzyme (pepsin) to begin protein digestion.
- Liver & Gall Bladder: The liver produces bile, which is stored in the gall bladder. Bile is released into the small intestine and has two key functions:
- Neutralisation: It is alkaline, neutralising the acidic mixture from the stomach to provide the optimum pH for enzymes in the small intestine.
- Emulsification: It breaks down large drops of fat into smaller droplets, increasing the surface area for lipase enzymes to act upon.
- Pancreas & Small Intestine: The pancreas produces protease, amylase, and lipase enzymes and releases them into the small intestine. The walls of the small intestine also produce enzymes. This is where the final digestion of proteins, carbohydrates, and fats occurs. The small intestine is also the primary site of absorption, being highly adapted with a huge surface area due to its length and the presence of villi and microvilli.
Concept 3: Enzymes as Biological Catalysts
Enzymes are proteins that act as biological catalysts, speeding up chemical reactions without being used up. The AQA specification places huge emphasis on the lock and key model.
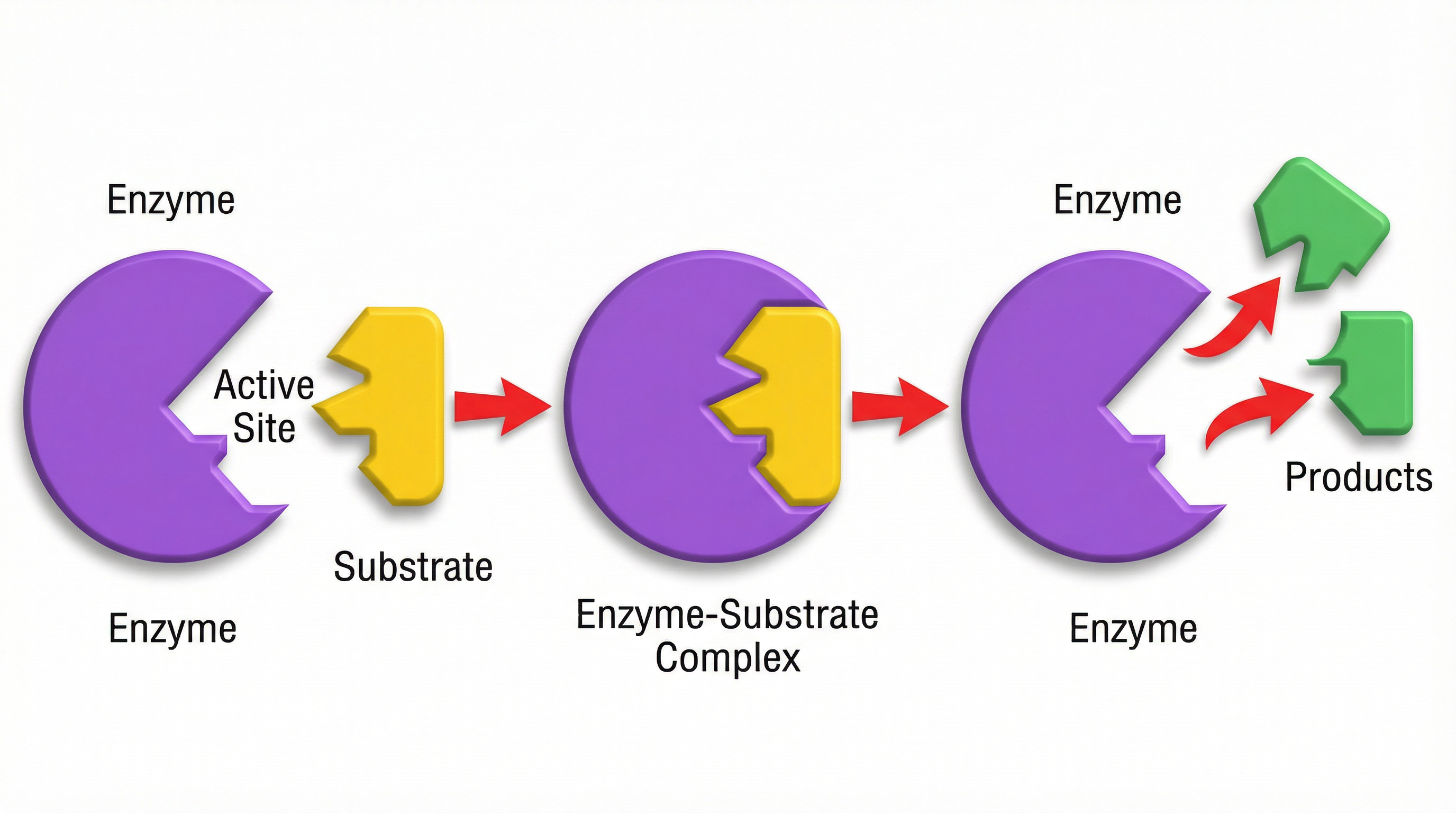
- Active Site: Each enzyme has a specific, three-dimensional shape, including a region called the active site.
- Specificity: The shape of the active site is complementary to the shape of only one type of substrate molecule. This is why enzymes are highly specific.
- Mechanism: The substrate (the key) fits into the active site (the lock). The enzyme then breaks the substrate down into products, which are released from the active site. The enzyme is then free to catalyse another reaction.
Factors Affecting Enzyme Action:
- Temperature: As temperature increases, the rate of reaction increases as enzymes and substrates have more kinetic energy and collide more frequently. However, beyond the optimum temperature, the bonds holding the enzyme in its specific shape begin to break. The active site changes shape, and the substrate no longer fits. The enzyme is denatured. This is an irreversible change.
- pH: A change in pH away from the optimum can also break the bonds within the enzyme, causing it to denature and lose its function.
Concept 4: The Circulatory System & The Heart
The circulatory system transports substances like oxygen, glucose, and waste products around the body. The heart acts as a powerful double pump.
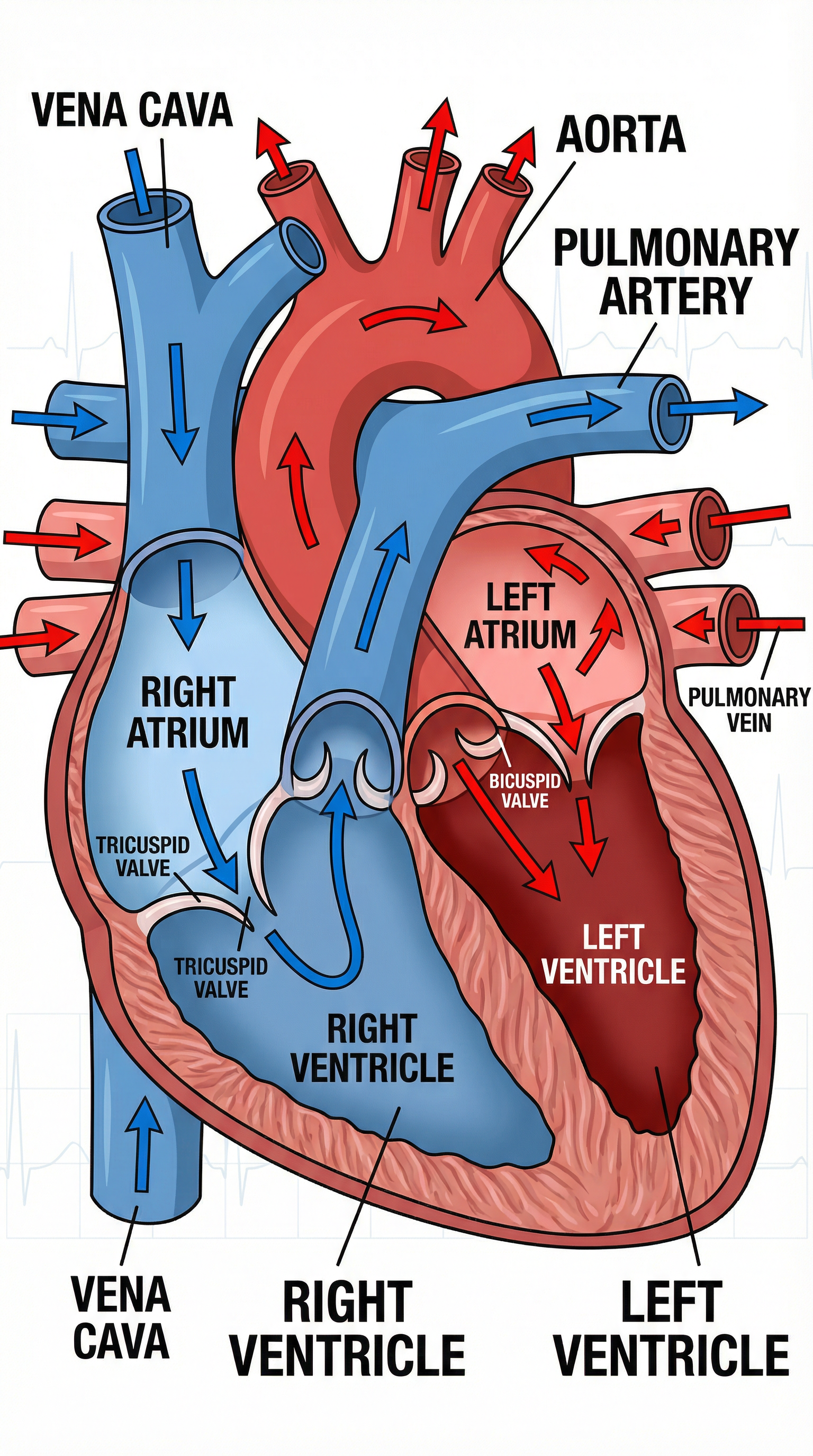
- Double Circulatory System: Humans have a double circulatory system:
- One circuit links the heart and lungs (the pulmonary circuit).
- The other circuit links the heart with the rest of the body (the systemic circuit).
- Blood Flow Path: You must be able to trace the path of blood. Deoxygenated blood enters the Right Atrium via the Vena Cava. It moves to the Right Ventricle and is pumped to the lungs via the Pulmonary Artery. Oxygenated blood returns from the lungs via the Pulmonary Vein into the Left Atrium. It moves to the Left Ventricle and is pumped to the rest of the body via the Aorta.
- Heart Structure & Function: A key marking point is explaining why the left ventricle has a thicker, more muscular wall than the right ventricle. This is because it needs to generate a much higher pressure to pump blood all around the body, whereas the right ventricle only needs to pump blood to the nearby lungs.
Mathematical/Scientific Relationships
While this topic is less formula-heavy, understanding rates of reaction is crucial for enzyme graph questions.
Rate of Reaction = (Change in amount of product or reactant) / (Time taken)
- Units: e.g., cm³/s or g/min.
- Application: This is used to analyse data from enzyme experiments (Required Practical 5). You might be asked to calculate the rate at the start of a reaction by drawing a tangent to the curve.
Practical Applications
Required Practical 4: Food Tests
Examiners will test your knowledge of the reagents and expected results for identifying biological molecules.
| Food Molecule | Reagent(s) | Preparation | Positive Result |
|---|---|---|---|
| Starch | Iodine solution | Add a few drops | Blue-black |
| Sugars (reducing) | Benedict's solution | Add and heat in a water bath | Brick-red (from blue) |
| Protein | Biuret solution | Add Biuret A then B | Purple/lilac |
| Lipids (Fats) | Sudan III stain | Add and shake | Red-stained oil layer |
Common Error: Forgetting that the Benedict's test for sugars requires heating in a water bath. No heat, no marks.
Required Practical 5: Effect of pH on Amylase
This practical investigates how pH affects the rate at which amylase breaks down starch.
- Method: Set up boiling tubes with starch solution and a buffer solution at different pH values. Add amylase and start a timer. Every 30 seconds, take a sample and add it to a drop of iodine on a spotting tile. Record the time taken for the iodine to no longer turn blue-black (indicating all starch has been broken down).
- Control Variables: Temperature (use a water bath), concentration of enzyme, concentration of starch.
- Graph Skills: You will be expected to plot the rate of reaction (1/time) against pH and identify the optimum pH.
