Study Notes
Overview
Welcome to the foundational topic of your GCSE Chemistry journey: Atoms, Elements, and Compounds. This unit, specification reference 4.1.1, is the bedrock upon which all other chemical principles are built. Understanding the nature of the atom is not just crucial for this topic but for grasping concepts like bonding, the periodic table, and chemical reactions later in the course. AQA examiners frequently test the historical evolution of the atomic model, so a firm grasp of the evidence from Rutherford's alpha scattering experiment is essential for securing top marks. Expect a range of question styles, from short-answer definitions to longer, 6-mark questions requiring detailed explanations and comparisons. This guide will equip you with the knowledge, exam technique, and memory aids to tackle this topic with confidence.
Key Concepts
Concept 1: The Structure of the Atom
At the heart of all matter is the atom, the smallest part of an element that can exist. It is composed of three subatomic particles: protons, neutrons, and electrons. Candidates must be able to recall the relative charges and masses of these particles to gain full credit in exams.
| Particle | Relative Mass | Relative Charge | Location in Atom |
|---|---|---|---|
| Proton | 1 | +1 | Nucleus |
| Neutron | 1 | 0 | Nucleus |
| Electron | Very small | -1 | Shells/Orbitals |
Key Point: Notice the mass of the electron is described as 'very small'. This is the precise language required by AQA mark schemes. Avoid using terms like 'negligible' or 'zero'.
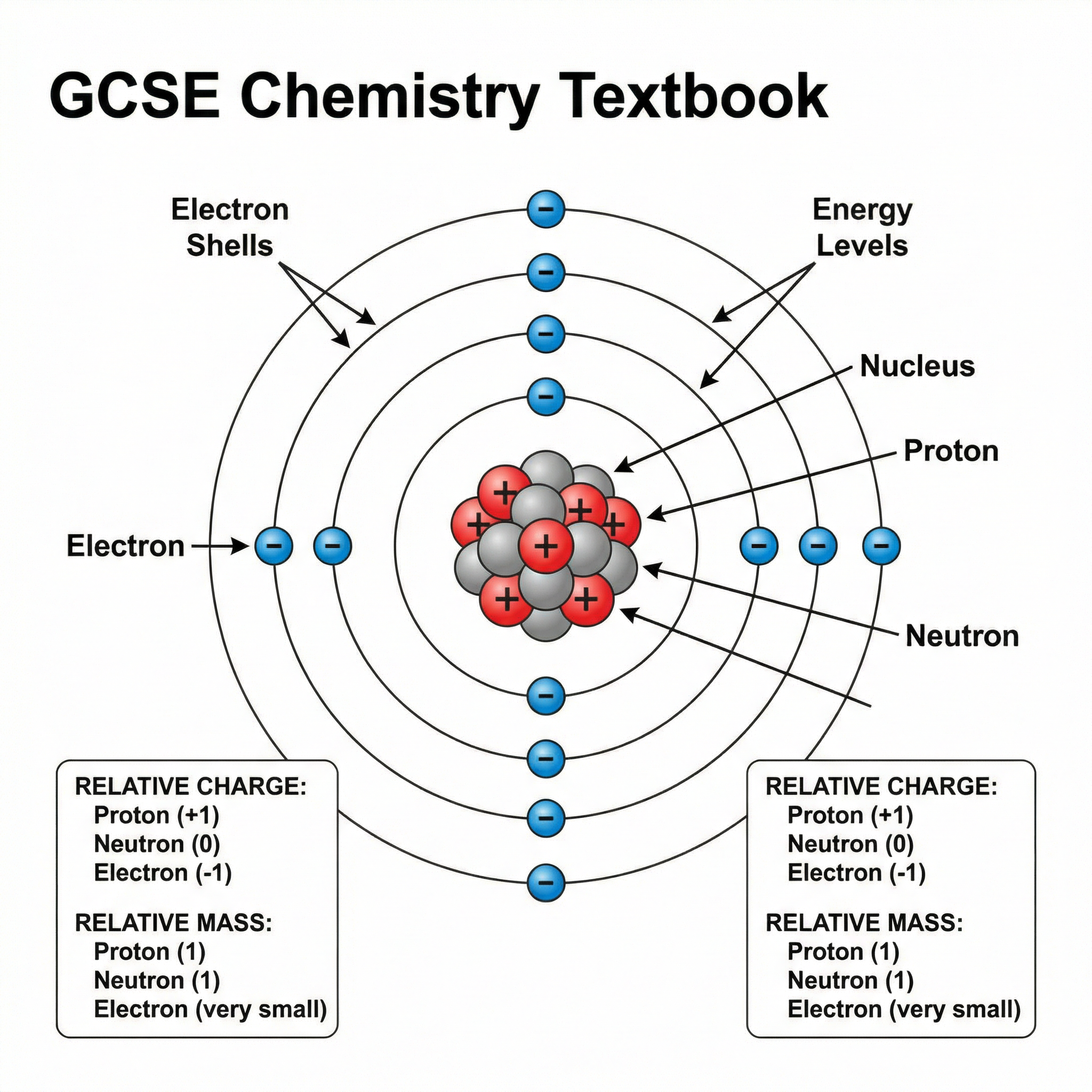
Concept 2: Atomic Number, Mass Number, and Isotopes
The identity of an element is determined by its atomic number (Z), which is the number of protons in the nucleus. The mass number (A) is the total number of protons and neutrons in the nucleus.
Isotopes are atoms of the same element (meaning they have the same number of protons) but with a different number of neutrons. For example, Carbon-12 has 6 protons and 6 neutrons, while Carbon-14 has 6 protons and 8 neutrons. This difference in neutron number affects the mass of the atom but not its chemical properties.
Concept 3: The Evolution of the Atomic Model
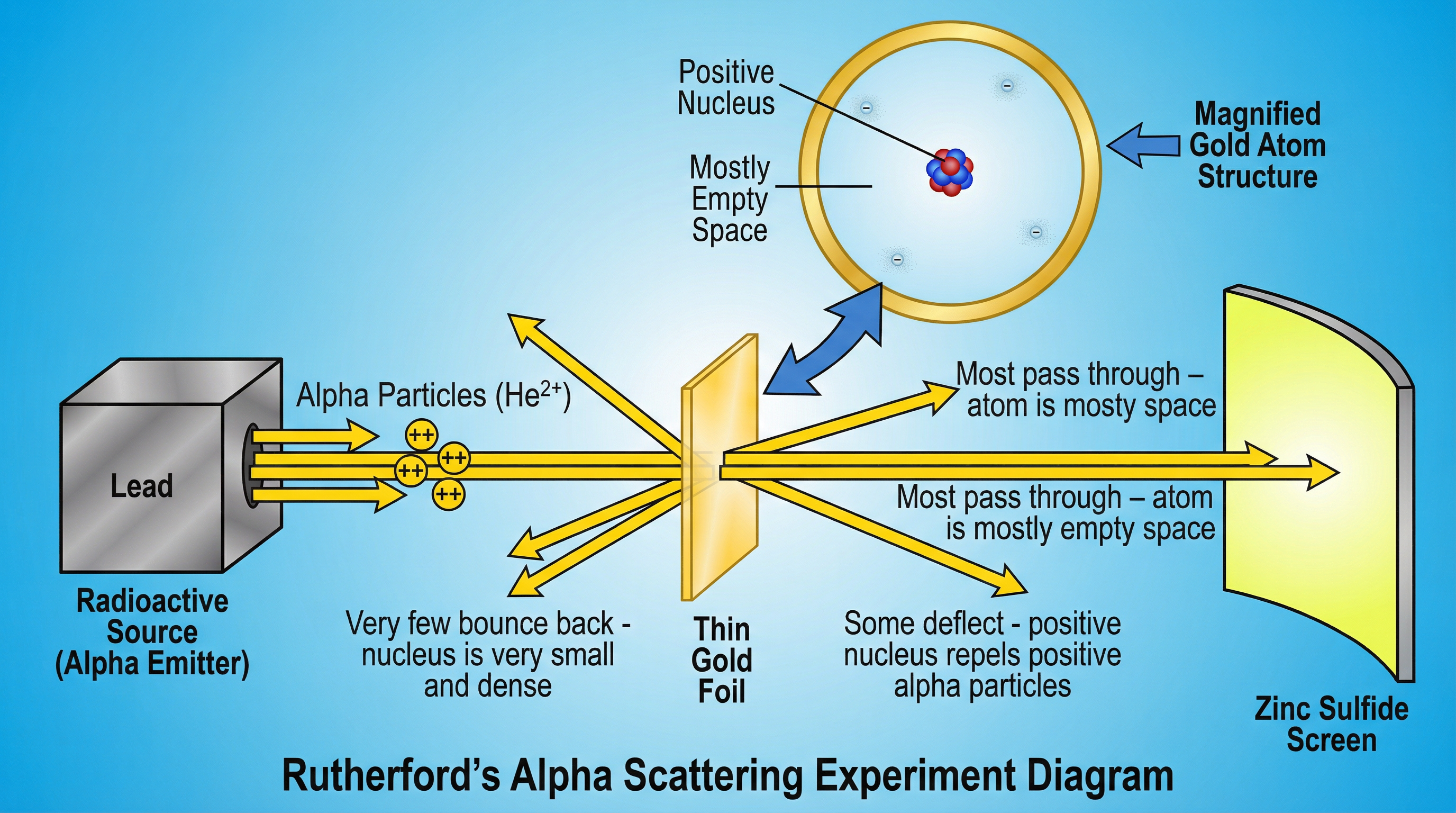
AQA places significant emphasis on the historical development of atomic theory. Candidates must be able to compare the 'plum pudding' model with the modern 'nuclear' model based on experimental evidence.
- Plum Pudding Model (pre-1909): Proposed the atom as a ball of positive charge with negative electrons embedded within it.
- Rutherford's Alpha Scattering Experiment: Alpha particles (positive charge) were fired at a thin gold foil.
- Observation 1: Most particles passed straight through. Conclusion: The atom is mostly empty space.
- Observation 2: Some particles were deflected. Conclusion: The nucleus has a positive charge, repelling the positive alpha particles.
- Observation 3: A very small number bounced back. Conclusion: The nucleus is very small, dense, and contains most of the atom's mass.
- Nuclear Model: This evidence led to the model of a small, dense, positive nucleus surrounded by orbiting electrons.
Concept 4: Elements, Compounds, and Mixtures
- Element: A substance consisting of only one type of atom (e.g., Oxygen, O).
- Compound: A substance formed when two or more different elements are chemically bonded together in fixed proportions (e.g., Water, H₂O). A chemical reaction is required to separate the elements in a compound.
- Mixture: Consists of two or more elements or compounds not chemically combined. The chemical properties of each substance in the mixture are unchanged. Mixtures can be separated by physical means.
Mathematical/Scientific Relationships
Calculating Relative Atomic Mass (Ar)
The relative atomic mass of an element is the weighted average mass of its isotopes relative to 1/12th the mass of a carbon-12 atom. For the exam, you must be able to calculate it from percentage abundances.
Formula (Must memorise):
Ar = [ (isotope mass A × % abundance A) + (isotope mass B × % abundance B) ] / 100
Example: Chlorine exists as two isotopes: Chlorine-35 (75% abundance) and Chlorine-37 (25% abundance).
Ar = [ (35 × 75) + (37 × 25) ] / 100 = (2625 + 925) / 100 = 3550 / 100 = 35.5
Practical Applications
Required Practical: Separation Techniques
This topic includes several key laboratory techniques that are frequently tested. Candidates must be familiar with the apparatus and method for each.
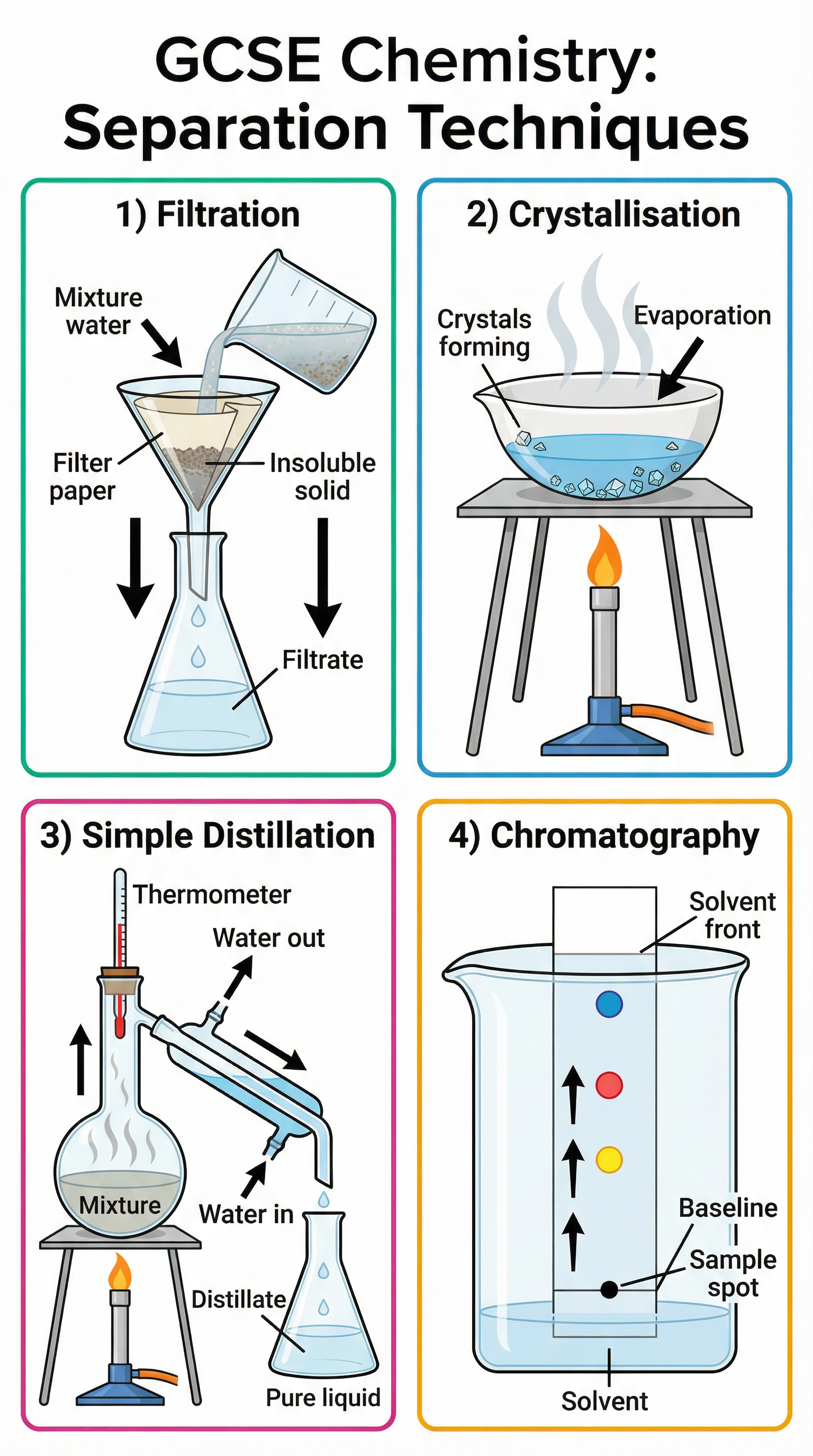
- Filtration: Used to separate an insoluble solid from a liquid (e.g., sand from water). The mixture is poured through filter paper in a funnel; the liquid (filtrate) passes through, and the solid (residue) is left behind.
- Crystallisation: Used to separate a soluble solid from a solution (e.g., salt from water). The solution is gently heated to evaporate some of the solvent, then left to cool. As it cools, the solid forms crystals.
- Simple Distillation: Used to separate a liquid from a solution (e.g., pure water from salt water). The solution is heated, the liquid with the lower boiling point evaporates, rises, cools in a condenser, and is collected as the distillate. Crucial detail: Water enters the condenser at the bottom and exits at the top to ensure the condenser jacket is always full, providing maximum cooling efficiency. Marks are consistently lost for incorrect water flow.
- Fractional Distillation: Used to separate a mixture of liquids with different boiling points (e.g., ethanol and water). It uses a fractionating column with a temperature gradient (hottest at the bottom, coolest at the top). The liquid with the lower boiling point evaporates first, rises highest, and is collected.
- Chromatography: Used to separate mixtures of soluble substances (e.g., different coloured inks). A spot of the mixture is placed on a baseline on chromatography paper, which is then placed in a solvent. The solvent moves up the paper, and the different substances travel at different rates, separating them out.
