Study Notes

Overview
Calculating Relative Formula Mass (Mr) is a fundamental skill in chemistry that underpins most quantitative work. It represents the combined mass of all atoms in a chemical formula, scaled relative to the mass of a carbon-12 atom. For your Edexcel GCSE exam, mastering Mr calculations is not just about one type of question; it is the gateway to accessing marks in a huge range of other topics, including moles, concentrations, percentage yield, and atom economy. Examiners frequently use Mr calculations as the first step in more complex, multi-stage problems. A mistake here can unfortunately lead to a cascade of lost marks, so getting it right is crucial. This guide will walk you through the process step-by-step, from simple molecules to complex hydrated salts, ensuring you can tackle any Mr question with confidence.
Key Concepts
Concept 1: Relative Atomic Mass (Ar)
Before you can calculate the Mr of a compound, you must understand Relative Atomic Mass (Ar). The Ar is the larger of the two numbers shown for an element on the Periodic Table provided in your exam. It represents the weighted average mass of the isotopes of an element compared to 1/12th the mass of a carbon-12 atom. For example, the Ar of Carbon (C) is 12, and the Ar of Chlorine (Cl) is 35.5. It is vital that candidates use this number, not the smaller Atomic (Proton) Number. A common error is to use the wrong value, which immediately results in zero marks for the calculation.
Example: For Magnesium (Mg), the Periodic Table shows two numbers: 12 and 24.3. The Relative Atomic Mass (Ar) is the larger one, 24.3 (often rounded to 24 for GCSE purposes unless specified otherwise).
Concept 2: The Summation Method
The core principle of calculating Mr is simple addition. You must identify every atom present in the chemical formula, find its Ar from the Periodic Table, and then sum these masses together. The process involves multiplying the Ar of each element by the number of atoms of that element in the formula (indicated by the subscript number).
Formula: Mr = Sum of (Number of atoms of an element × Ar of the element)
Example: For Carbon Dioxide (CO2):
- One Carbon atom: 1 × 12 = 12
- Two Oxygen atoms: 2 × 16 = 32
- Total Mr = 12 + 32 = 44
Concept 3: Handling Brackets
This is a key area where marks are often lost, particularly at the Higher tier. When a formula contains brackets, the small number (subscript) outside the bracket multiplies every atom inside the bracket. Candidates must apply this multiplier correctly to get the right atom count before summing the masses.
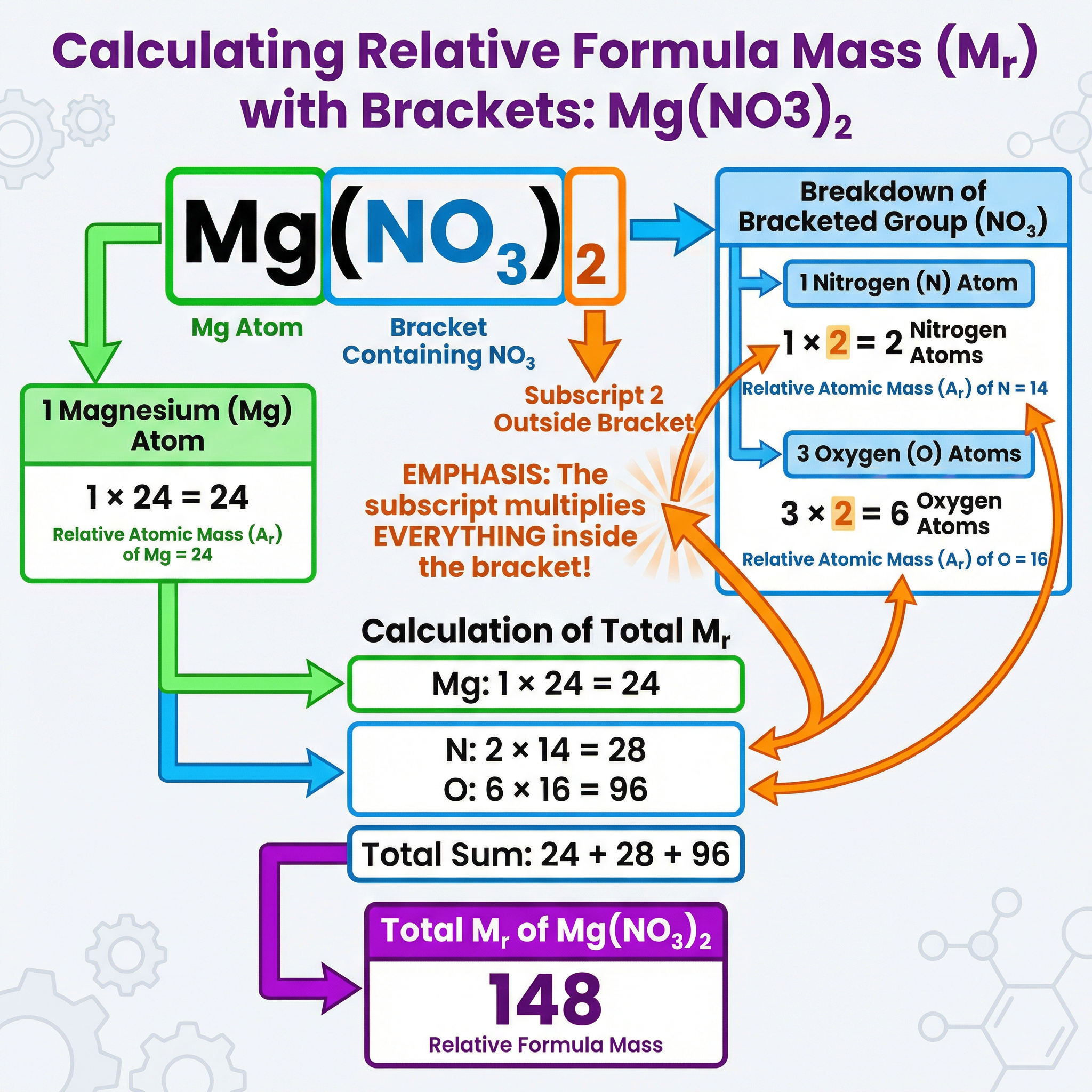
Example: For Calcium Hydroxide, Ca(OH)2:
- The (OH) group is in brackets with a subscript 2 outside.
- This means there are 2 Oxygen atoms and 2 Hydrogen atoms.
- One Calcium atom: 1 × 40 = 40
- Two Oxygen atoms: 2 × 16 = 32
- Two Hydrogen atoms: 2 × 1 = 2
- Total Mr = 40 + 32 + 2 = 74
Concept 4: Hydrated Salts (Higher Tier)
Hydrated salts contain water of crystallisation, indicated by a dot in the formula (e.g., ·5H2O). This dot signifies addition, not multiplication. To calculate the Mr of a hydrated salt, you calculate the Mr of the anhydrous salt part and then add the total mass of the water molecules.
Example: For hydrated Copper(II) Sulfate, CuSO4·5H2O:
- First, calculate the Mr of the anhydrous salt, CuSO4:
- Cu: 1 × 64 = 64
- S: 1 × 32 = 32
- O: 4 × 16 = 64
- Mr of CuSO4 = 64 + 32 + 64 = 160
- Next, calculate the total mass of the water molecules, 5H2O:
- Mr of H2O = (2 × 1) + 16 = 18
- Mass of 5 water molecules = 5 × 18 = 90
- Finally, add the two parts together:
- Total Mr = 160 + 90 = 250
