Study Notes
Overview
Chromatography is a powerful analytical technique used to separate and identify substances within a mixture. For your AQA GCSE Chemistry exam, understanding chromatography is crucial as it's a guaranteed topic, especially through questions on Required Practical 6. This guide will break down the process into simple, memorable steps, explain the science behind it, and show you how to apply your knowledge to exam-style questions. We'll explore how substances are separated based on their distribution between a stationary phase (the paper) and a mobile phase (the solvent), and how to use Rf values to identify them. Mastering this topic will not only prepare you for the exam but also provide a solid foundation for A-Level Chemistry.
Key Concepts
Concept 1: Phases and Separation
At its heart, chromatography is a story of two phases: the stationary phase and the mobile phase. In paper chromatography, the stationary phase is the chromatography paper, and the mobile phase is the solvent that moves up the paper. The separation of a mixture into its components depends on how each component distributes itself between these two phases.
- High solubility in mobile phase + Low attraction to stationary phase = Travels far up the paper.
- **Low solubility in mobile phase + High attraction to stationary phase = Travels a short distance up the paper.**This differential movement is the key to separation. Imagine a race where some runners are more drawn to the track (stationary phase) while others are more carried along by a moving walkway (mobile phase). The ones on the walkway will travel furthest!
Concept 2: Required Practical 6 - Method and Common Errors
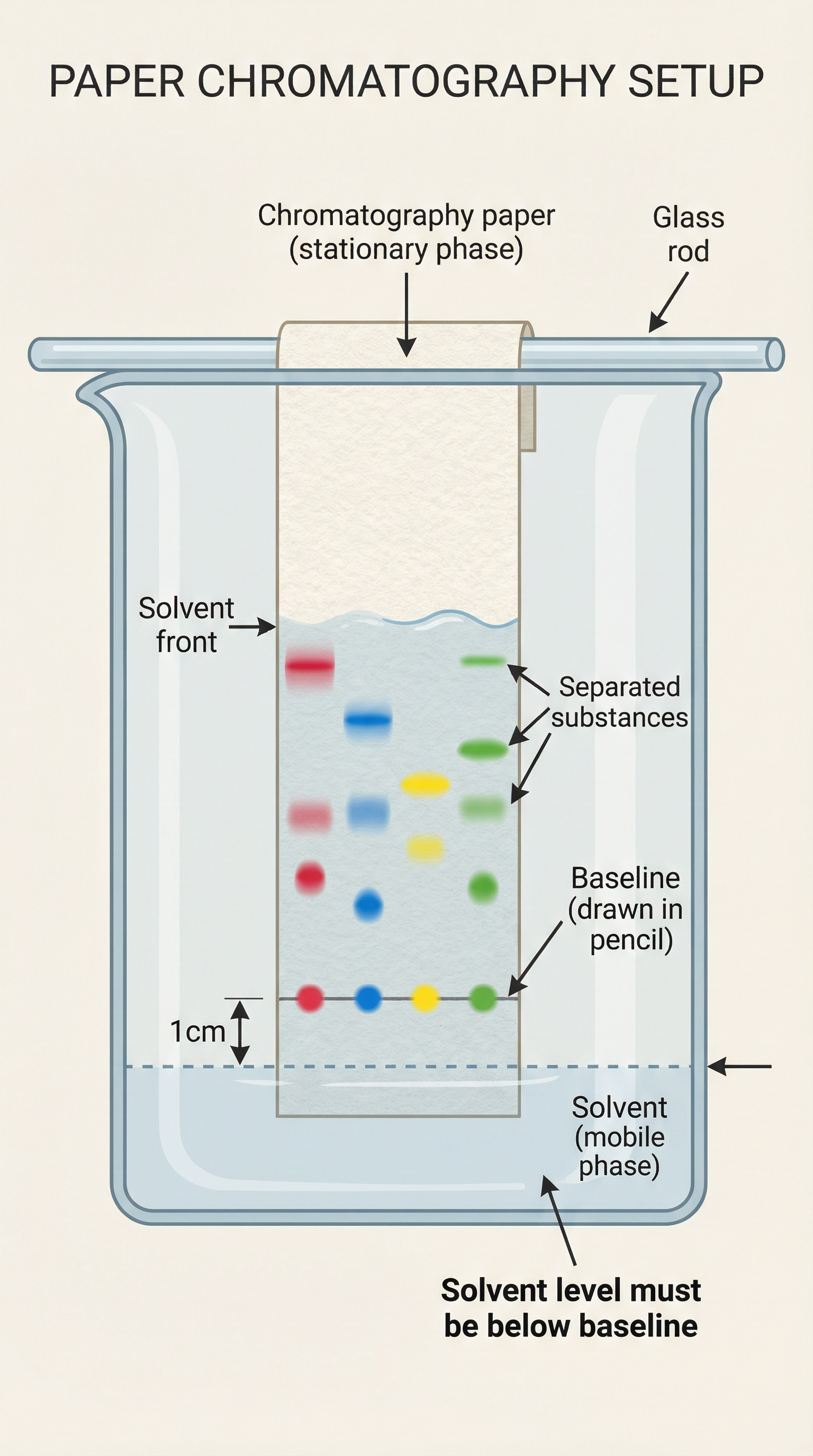
The AQA exam board loves to test your practical skills. Here's a breakdown of the method for paper chromatography:
- Draw a baseline: Use a pencil to draw a straight line about 1-2 cm from the bottom of the chromatography paper. Crucially, it must be pencil. Graphite is insoluble in the solvent, whereas the ink from a pen would dissolve and interfere with the results. This is a classic exam question!
- Spot the samples: Place a small, concentrated spot of each sample onto the baseline.
- Place in solvent: Suspend the paper in a beaker containing a small amount of solvent, ensuring the solvent level is below the baseline. If the solvent covers the baseline, the samples will dissolve into the solvent at the bottom of the beaker instead of travelling up the paper.
- Allow separation: Cover the beaker with a lid (e.g., a watch glass) to create a saturated atmosphere and prevent the solvent from evaporating. Allow the solvent to travel up the paper.
- Mark the solvent front: Once the solvent has travelled most of the way up the paper, remove it from the beaker and immediately mark the position of the solvent front with a pencil.
- Calculate Rf values: Measure the distance travelled by each spot and by the solvent, then use the Rf formula.
Mathematical/Scientific Relationships
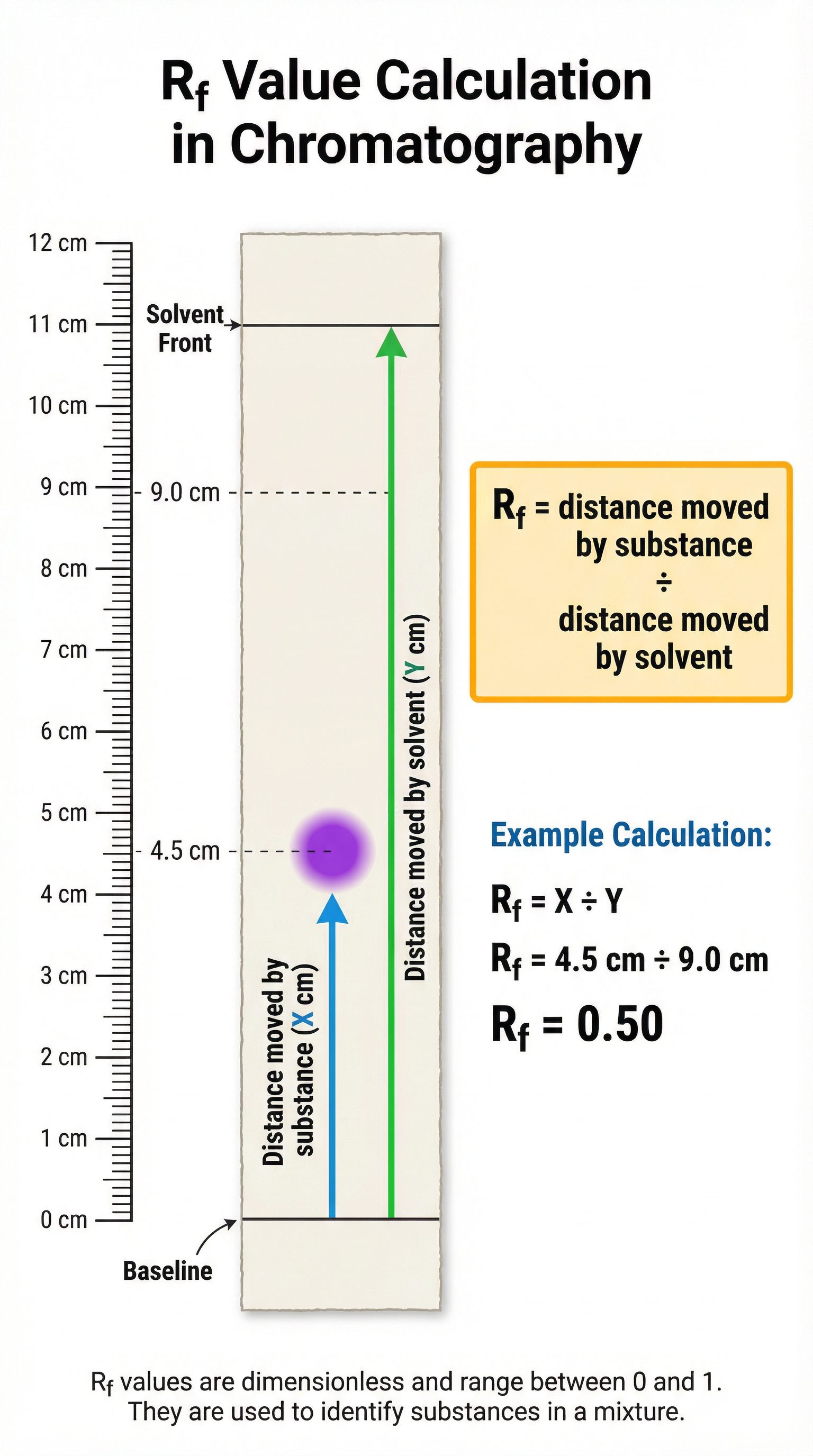
The Rf Value Formula
The Rf (Retention factor) value is a ratio that is constant for a particular substance in a given solvent, under the same conditions. It allows you to identify unknown substances by comparing their Rf values to those of known substances.
Formula:
Rf = Distance moved by substance / Distance moved by solvent
- This formula must be memorised. It is not given on the formula sheet.
- Distance moved by substance: Measured from the baseline to the centre of the spot.
- Distance moved by solvent: Measured from the baseline to the solvent front.
- Rf values are always between 0 and 1 and have no units.
Practical Applications
Chromatography isn't just a classroom experiment; it has many real-world applications:
- Forensic Science: To identify substances at a crime scene, such as drugs or explosives.
- Food Industry: To separate the components of food colourings to check for illegal additives.
- Pharmaceuticals: To ensure the purity of drugs.
- Medical Testing: To test for drugs in urine samples.