Study Notes
Overview
The Periodic Table is the definitive map of the chemical elements and one of the most powerful tools in chemistry. For your OCR GCSE exam, a deep understanding of its structure and the patterns it reveals is essential for success. This topic is not just about memorising element positions; it's about understanding the fundamental principles of atomic structure that dictate an element's behaviour. You will be expected to link an element's position to its electron configuration and use this to explain trends in properties like reactivity. Typical exam questions range from simple recall of definitions to extended 6-mark questions requiring detailed explanations of reactivity trends in Groups 1 and 7. Mastering this topic provides a solid foundation for understanding bonding, energy changes, and chemical reactions later in the course.
Key Concepts
Concept 1: The Structure of the Periodic Table
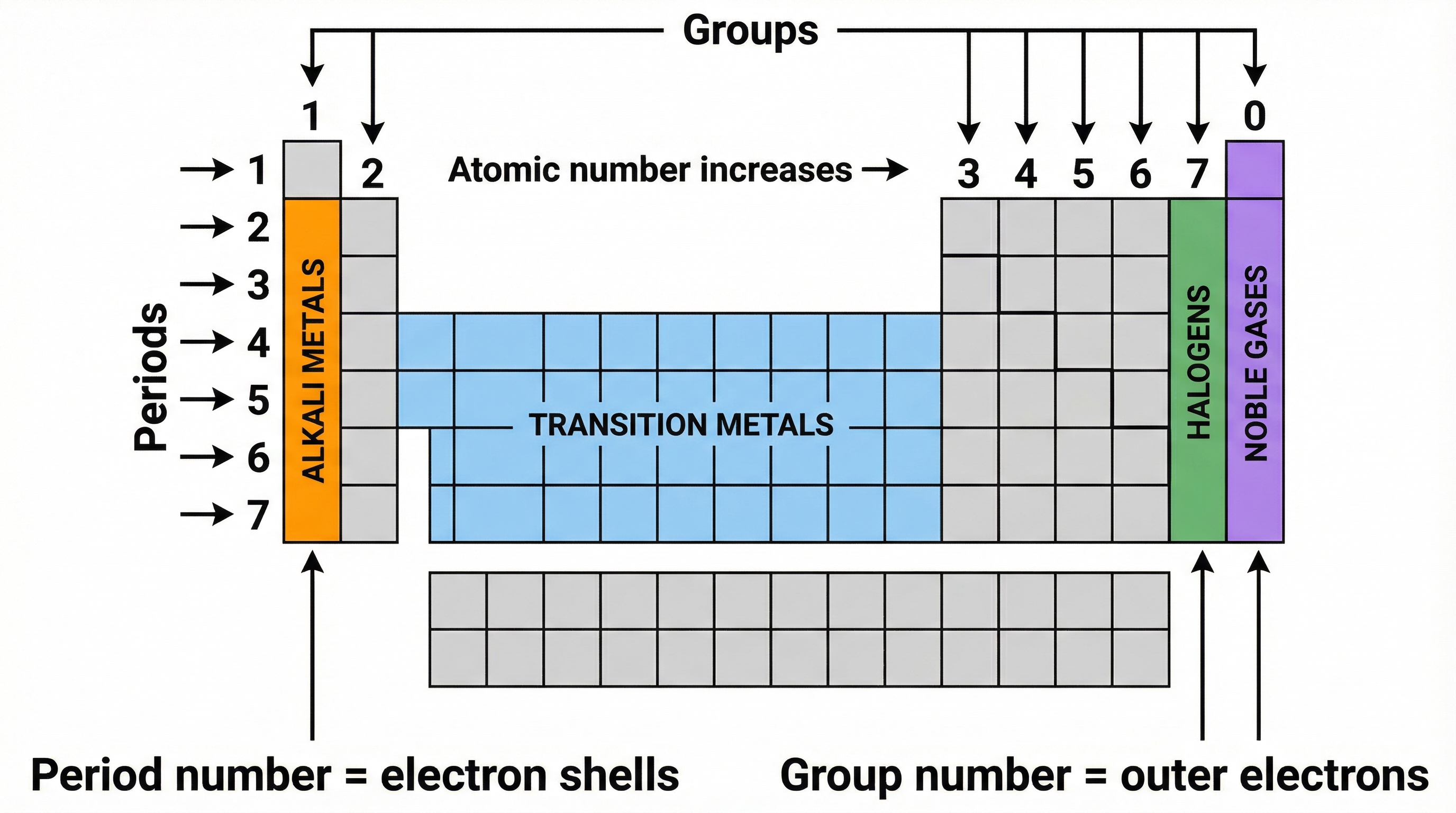
The modern Periodic Table arranges elements in order of increasing atomic number. The atomic number represents the number of protons in an atom's nucleus, which uniquely defines an element. The table is organised into vertical columns called groups and horizontal rows called periods.
- Periods: The period number (from 1 to 7) tells you the number of electron shells an atom of that element has. For example, Sodium (Na) is in Period 3, so it has three electron shells.
- Groups: For the main group elements (Groups 1, 2, and 13-18 or 0), the group number indicates the number of electrons in the outermost shell (valence electrons). For example, Chlorine (Cl) is in Group 7, so it has seven outer electrons. This is the key to an element's chemical properties.
Concept 2: The History of the Periodic Table - Mendeleev's Contribution
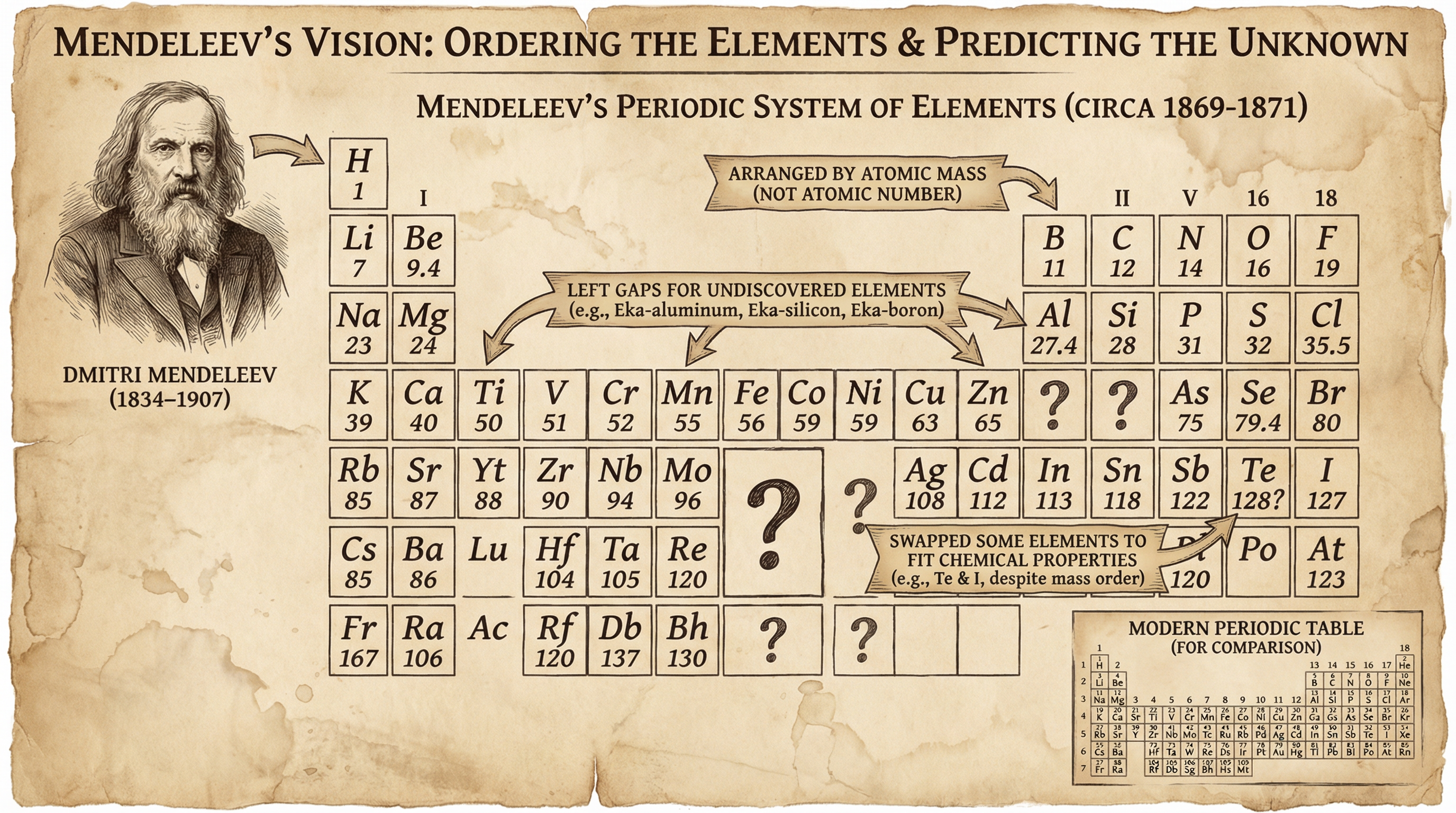
Before the discovery of protons and atomic numbers, scientists like Dmitri Mendeleev attempted to organise the elements. In 1869, Mendeleev arranged the known elements by atomic mass. His genius lay in two key decisions:
- Leaving Gaps: He recognised that some elements were missing and left gaps for them, predicting their properties with remarkable accuracy (e.g., Eka-aluminium, which we now know as Gallium).
- Swapping Elements: He swapped the order of some elements (like Tellurium and Iodine) if their chemical properties fitted better with a different group, prioritising chemical behaviour over strict atomic mass order.
This was a crucial step, but the modern table is ordered by atomic number, which resolves the issues Mendeleev encountered.
Concept 3: Reactivity Trends in Group 1 (The Alkali Metals)
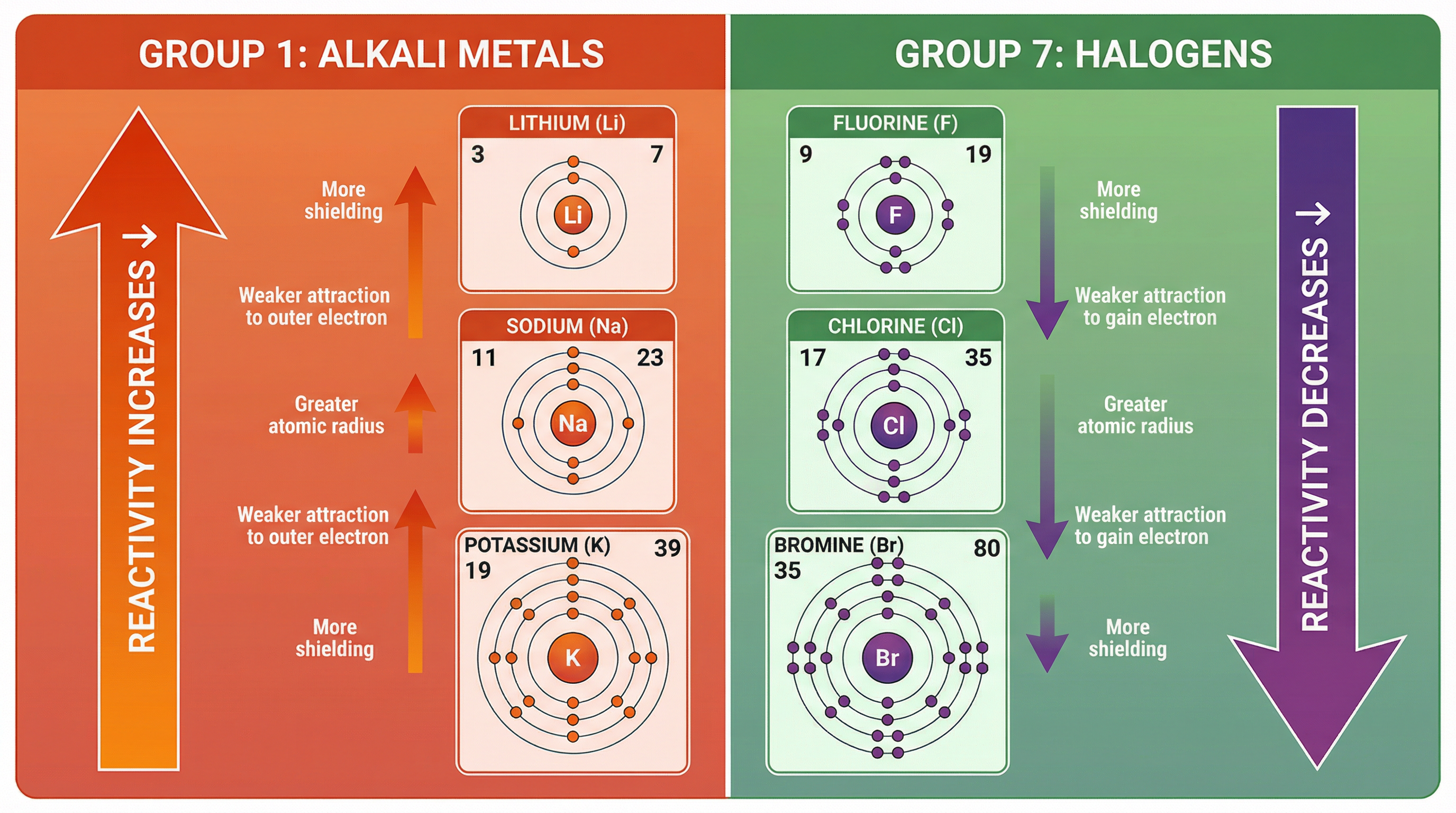
Group 1 elements (Lithium, Sodium, Potassium etc.) are highly reactive metals. Their reactivity increases as you go down the group. This is because they react by losing one electron from their outer shell to form a +1 ion. The easier it is to lose this electron, the more reactive the metal.
As you go down Group 1:
- Atomic Radius Increases: Each element has an extra electron shell, so the atom gets bigger.
- Shielding Increases: There are more inner shells of electrons to shield the outer electron from the nucleus's pull.
- Weaker Electrostatic Attraction: The outer electron is further from the nucleus and more shielded, so the electrostatic force of attraction between the positive nucleus and the negative outer electron is weaker.
Conclusion: Less energy is needed to remove the outer electron, making the element more reactive.
Concept 4: Reactivity Trends in Group 7 (The Halogens)
The Halogens (Fluorine, Chlorine, Bromine etc.) are reactive non-metals. Their reactivity decreases as you go down the group. This is because they react by gaining one electron to complete their outer shell, forming a -1 ion. The easier it is to attract and gain an electron, the more reactive the non-metal.
As you go down Group 7:
- Atomic Radius Increases: Atoms get bigger with more shells.
- Shielding Increases: More inner shells shield the nucleus.
- Weaker Electrostatic Attraction: The nucleus finds it harder to attract an incoming electron into the outer shell because it is further away and more shielded.
Conclusion: The ability to attract an electron decreases, making the element less reactive.
Concept 5: Group 0 (The Noble Gases)
Group 0 elements (Helium, Neon, Argon etc.) are extremely unreactive, or inert. This is because they have a full outer shell of electrons. This is a very stable electronic arrangement, so they have no tendency to lose, gain, or share electrons to form bonds. This is the key phrase examiners look for!
Mathematical/Scientific Relationships
There are no complex formulas to memorise for this topic. The key relationships are conceptual:
- Group Number = Number of Outer Shell Electrons
- Period Number = Number of Occupied Electron Shells
Practical Applications
- Alkali Metals: Used in streetlights (Sodium), batteries (Lithium), and fertilisers (Potassium).
- Halogens: Used in disinfectants and bleach (Chlorine), toothpaste (Fluoride), and flame retardants (Bromine).
- Noble Gases: Used in light bulbs (Argon), lasers (Helium-Neon), and party balloons (Helium).