Study Notes
Overview
Condensation polymerisation is a fundamental process in organic chemistry where monomers join together to form a large polymer chain with the simultaneous release of a small molecule, such as water. For AQA GCSE Chemistry, this topic (specification reference 7.7) is exclusive to the Higher Tier and focuses specifically on the formation of polyesters from the reaction between dicarboxylic acids and diols. Unlike addition polymerisation, which involves the opening of a C=C double bond, this mechanism is all about the reaction between two different functional groups. Understanding this distinction is critical for exam success, as comparison questions are extremely common and carry significant marks. This guide will break down the mechanism, show you how to draw the structures examiners expect, and provide strategies to tackle the toughest questions.
Key Concepts
Concept 1: The Monomers - Two is the Magic Number
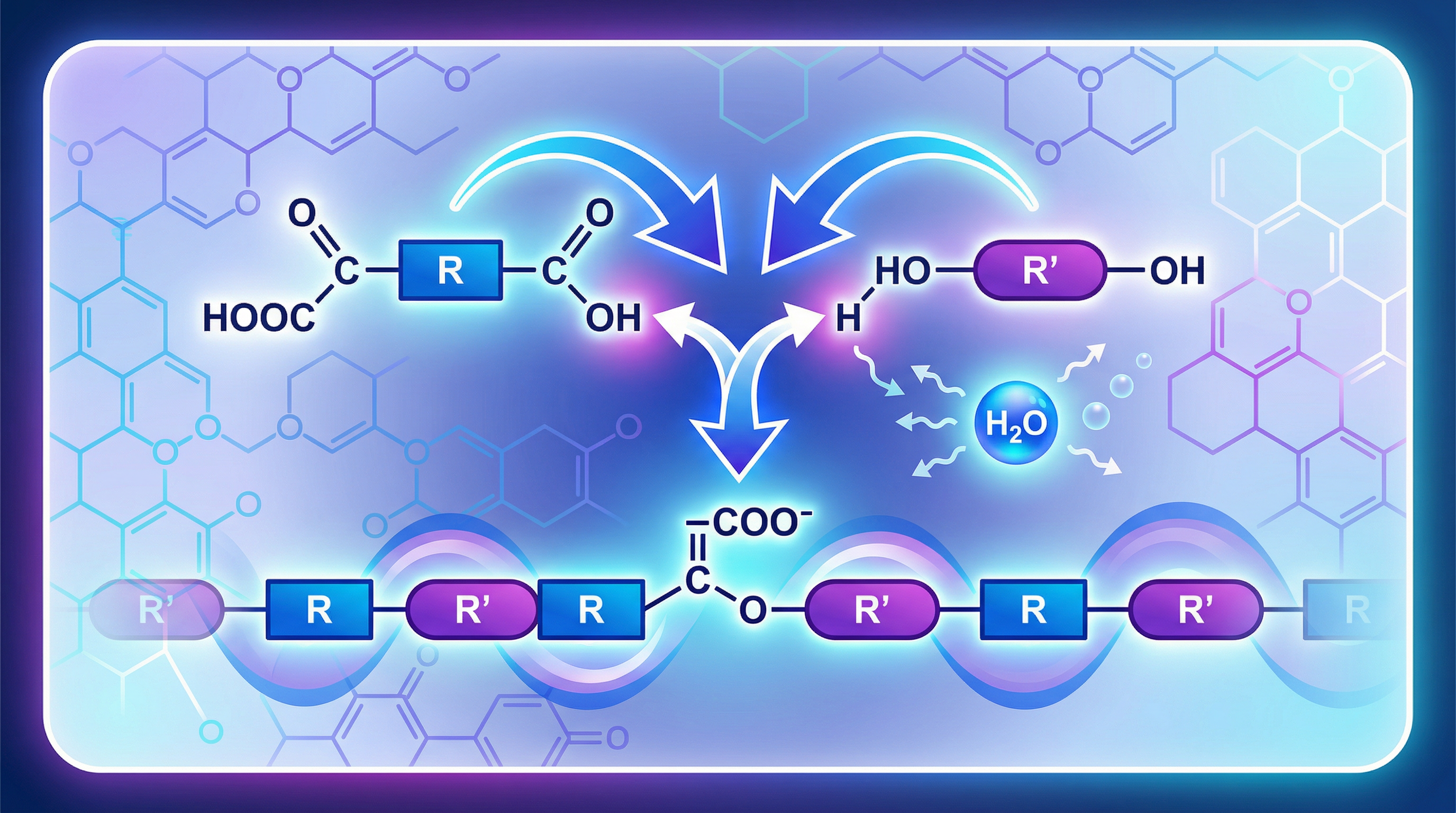
Credit is always awarded for stating that condensation polymerisation requires monomers with two functional groups. This is the absolute cornerstone of the topic. For polyester formation, the two types of monomers are:
- Dicarboxylic Acids: These are molecules containing two carboxylic acid functional groups (-COOH), one at each end. For example, ethanedioic acid (HOOC-COOH).
- Diols: These are molecules containing two alcohol functional groups (-OH), one at each end. For example, ethane-1,2-diol (HO-CH₂-CH₂-OH).
The presence of two reactive sites on each monomer allows the chain to grow in both directions, leading to the formation of a long polymer.
Concept 2: The Reaction - Forming the Ester Link
The reaction occurs between the carboxylic acid group of one monomer and the alcohol group of the other. Specifically, the -OH part of the carboxylic acid group reacts with the -H from the alcohol group. These atoms combine and are eliminated as a water molecule (H₂O). The remaining parts of the monomers join together, forming an ester linkage (-COO-). This is the repeating link that holds the entire polymer chain together.
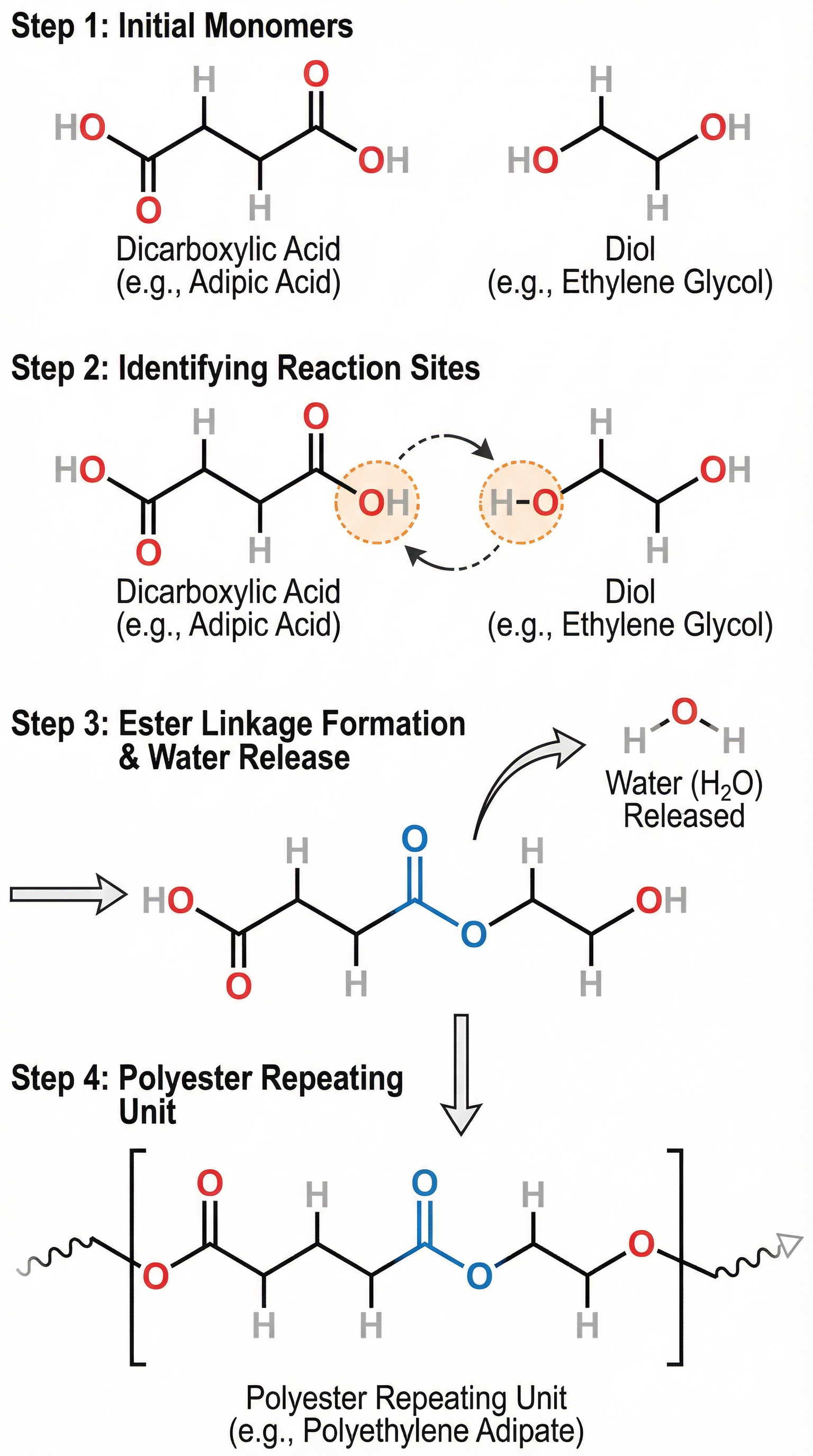
Concept 3: The Products - Polymer and a Small Molecule
A key difference that you must be able to recall is that condensation polymerisation produces two products:
- The Polymer: A long-chain molecule (in this case, a polyester).
- A Small Molecule: For the formation of polyesters, this is always water (H₂O).
This is in direct contrast to addition polymerisation, which only forms one product (the polymer). Being able to clearly articulate this difference is a frequent source of marks.
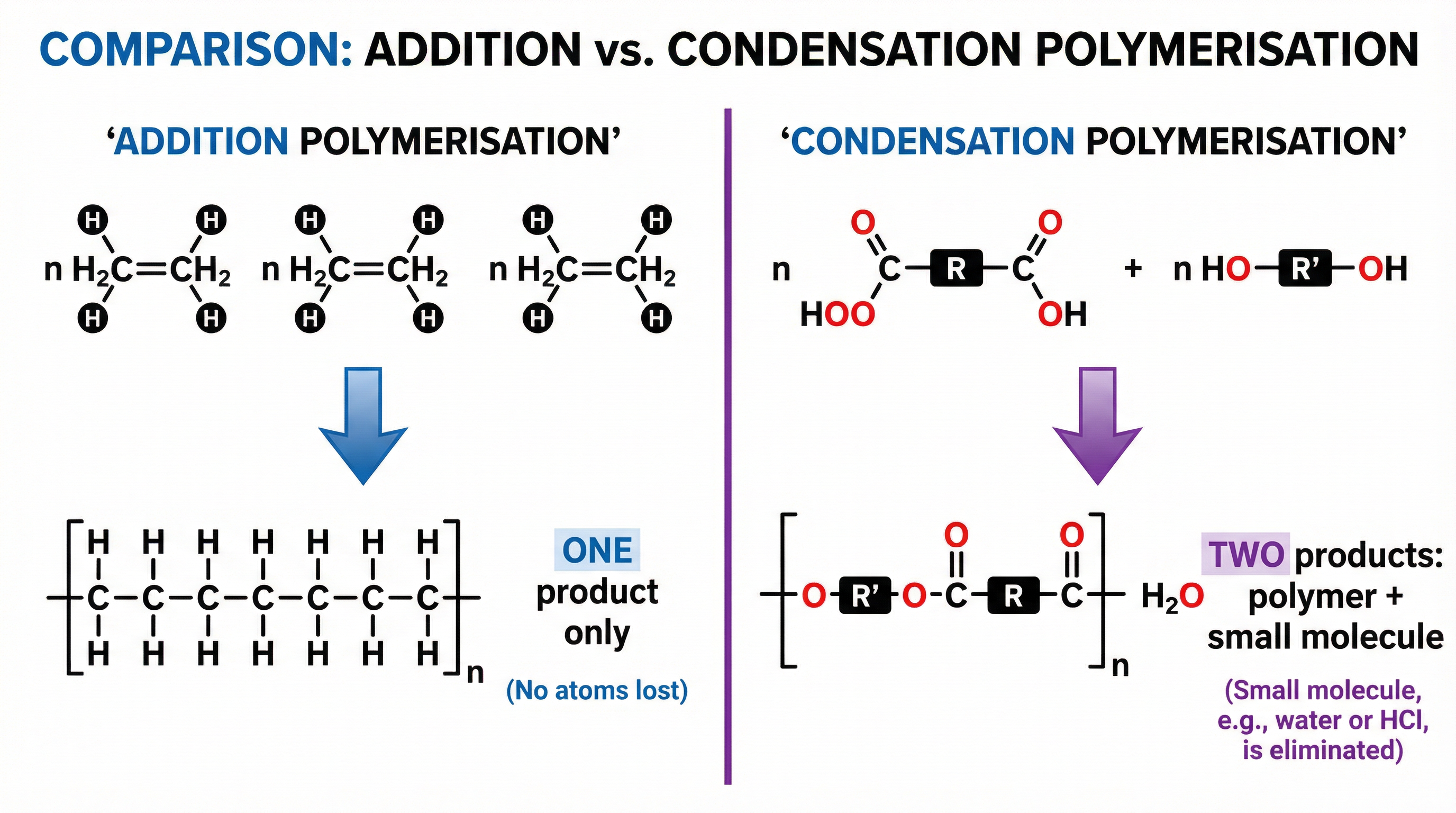
Mathematical/Scientific Relationships
The general equation for the formation of a polyester is a crucial piece of knowledge that combines all the key concepts. When 'n' molecules of a dicarboxylic acid react with 'n' molecules of a diol, they produce a polymer chain of 'n' repeating units and, importantly, '2n' molecules of water.
**General Equation:**n HOOC-R-COOH + n HO-R'-OH → [-OC-R-COO-R'-]n + 2n H₂O
- n: Represents a large number, indicating many monomer units.
- R and R': Represent the hydrocarbon sections of the monomers.
- [-OC-R-COO-R'-]n: This is the repeating unit of the polyester. Note the ester links at both ends of the R groups and the extension bonds passing through the brackets.
- 2n H₂O: This is the part candidates often forget. For every repeating unit formed, two ester links are effectively made (one at each end joining to the next monomer), and thus two molecules of water are eliminated. The total water produced is 2n.
Practical Applications
Polyesters are incredibly versatile polymers with widespread applications. The most well-known example is poly(ethylene terephthalate), commonly known as PET. It is used to make plastic bottles for drinks due to its strength, transparency, and unreactivity. Polyesters can also be drawn into fibres to produce fabrics for clothing, such as fleece jackets and sportswear. These materials are popular because they are lightweight, durable, and resistant to stretching and shrinking. They also dry quickly, making them ideal for outdoor wear.