Study Notes
Overview
Welcome to the core of chemistry: Atomic Structure. This topic is fundamental, not just for passing your GCSE, but for understanding almost every other concept in chemistry, from bonding to the periodic table. Edexcel examiners focus on two key areas: your ability to recall the properties of subatomic particles and the historical development of the atomic model, and your skill in applying mathematical principles to calculate relative atomic mass. Expect to see a mix of short-answer questions demanding precise definitions and longer, 6-mark questions that require you to compare and contrast different scientific ideas. This guide will equip you with the knowledge and exam technique to secure every available mark.
Key Concepts
Concept 1: Subatomic Particles
At the heart of every atom are three fundamental subatomic particles: protons, neutrons, and electrons. Your exam success hinges on knowing their properties inside out. The nucleus, a tiny, dense core at the atom's centre, contains the protons and neutrons, giving it a positive charge and accounting for almost all the atom's mass. The electrons are found in specific energy levels, or shells, orbiting the nucleus.
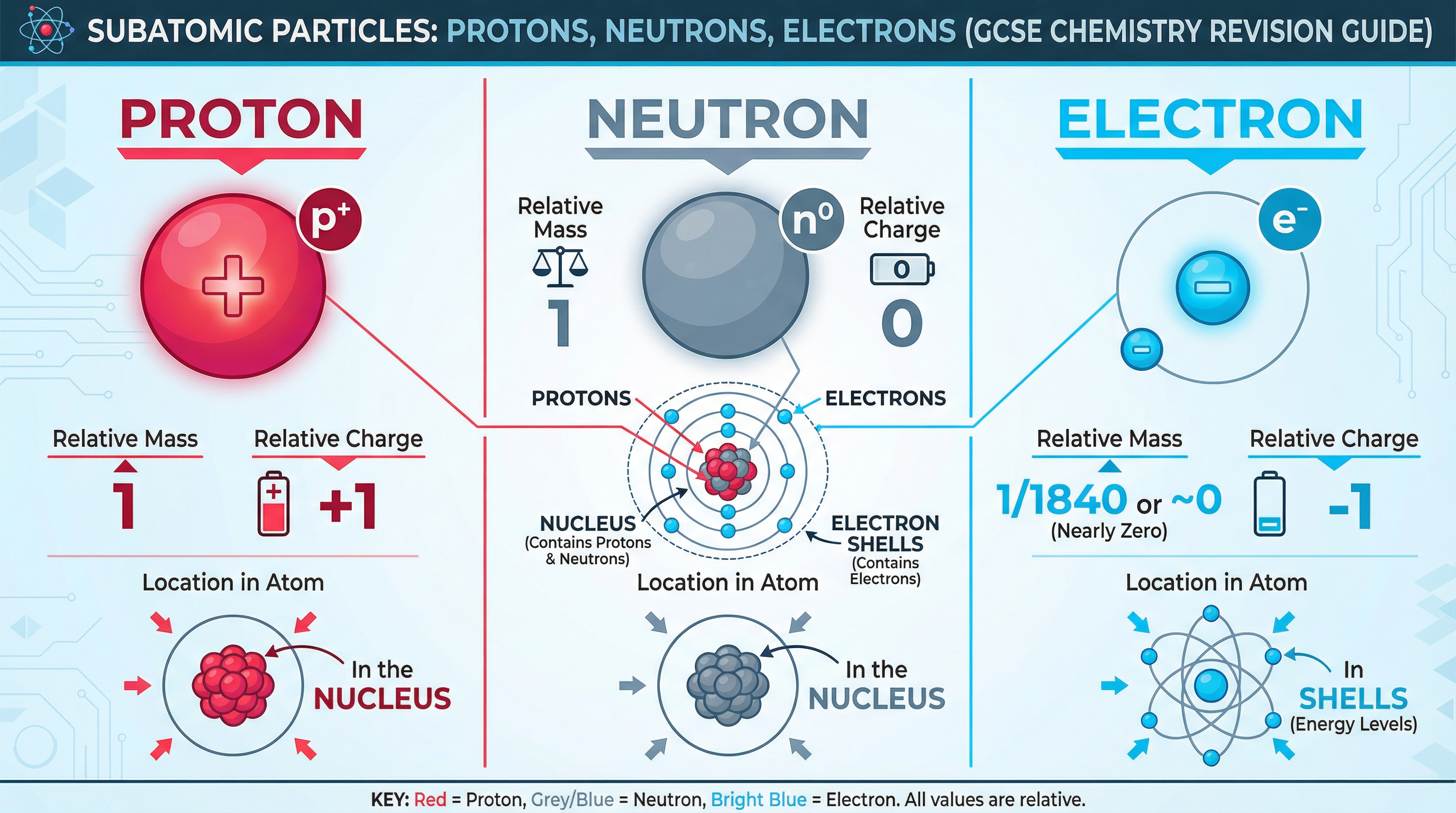
| Particle | Relative Mass | Relative Charge | Location in Atom |
|---|---|---|---|
| Proton | 1 | +1 | Nucleus |
| Neutron | 1 | 0 | Nucleus |
| Electron | ~0 (1/1840) | -1 | Shells / Energy Levels |
Why it works: The number of protons (the Atomic Number) defines the element. A neutral atom has an equal number of protons and electrons, balancing the overall charge to zero. The total number of protons and neutrons gives the Mass Number.
Example: A sodium atom has the symbol Na with numbers 23 and 11. This means it has 11 protons, 11 electrons (in a neutral atom), and 23 - 11 = 12 neutrons.
Concept 2: The Evolution of the Atomic Model
Scientific understanding of the atom has changed over time. Examiners expect you to compare the Plum Pudding model with the modern Nuclear model, often in 6-mark questions. This is a story of evidence leading to new theories.
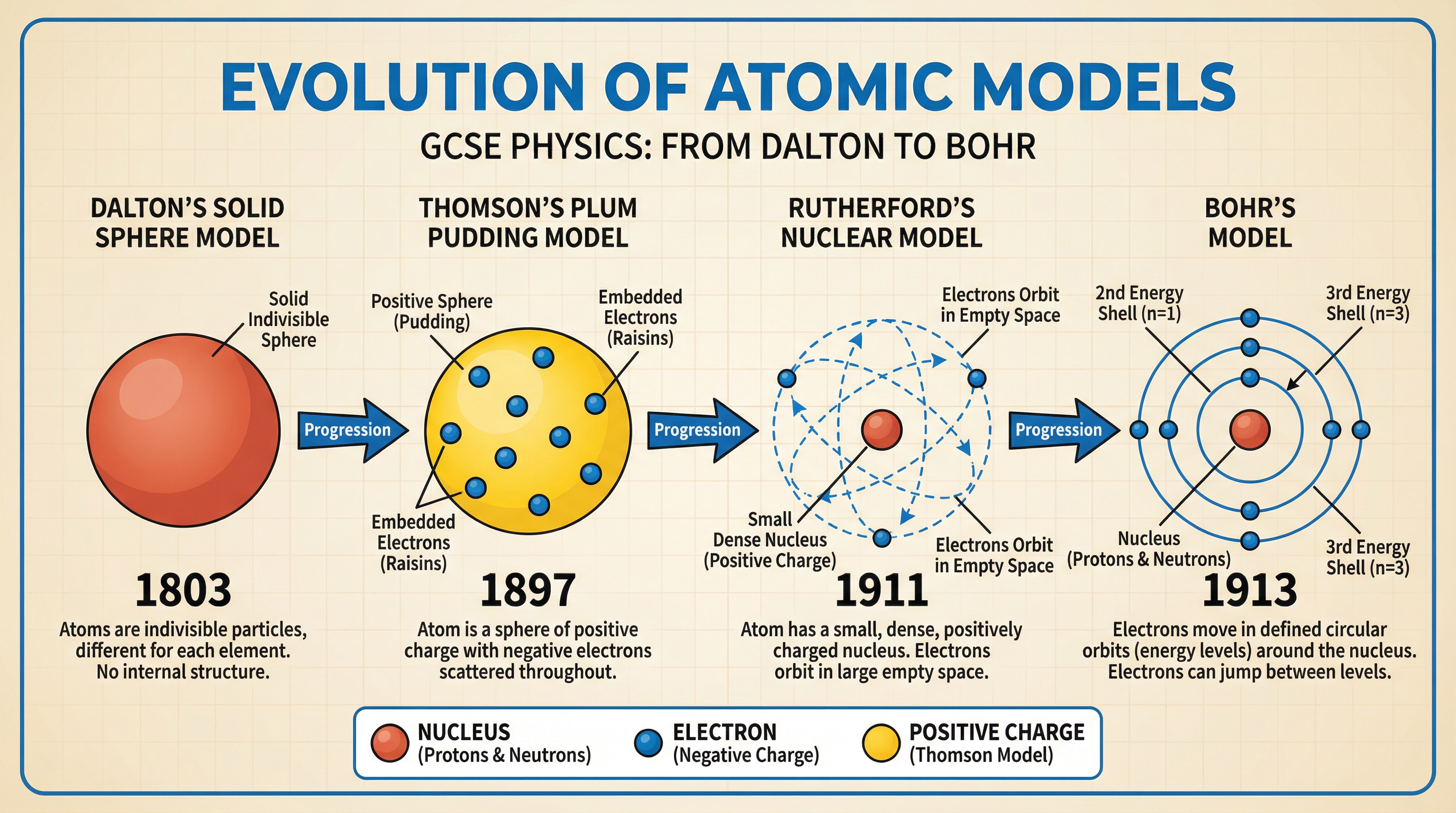
- Dalton's Model (early 1800s): Atoms were imagined as tiny, solid, indivisible spheres.
- Thomson's Plum Pudding Model (1897): After discovering the electron, J.J. Thomson proposed the atom was a sphere of positive charge with negative electrons dotted throughout, like plums in a pudding. There was no nucleus.
- Rutherford's Nuclear Model (1911): The alpha particle scattering experiment provided crucial evidence against the Plum Pudding model. Most alpha particles passed straight through a thin gold foil, showing the atom is mostly empty space. The deflection of a few particles revealed a tiny, dense, positively-charged nucleus at the centre.
- Bohr's Model (1913): Niels Bohr refined Rutherford's model by suggesting electrons orbit the nucleus in fixed energy levels or shells. This explained why atoms emit light at specific frequencies.
Concept 3: Isotopes and Relative Atomic Mass (Ar)
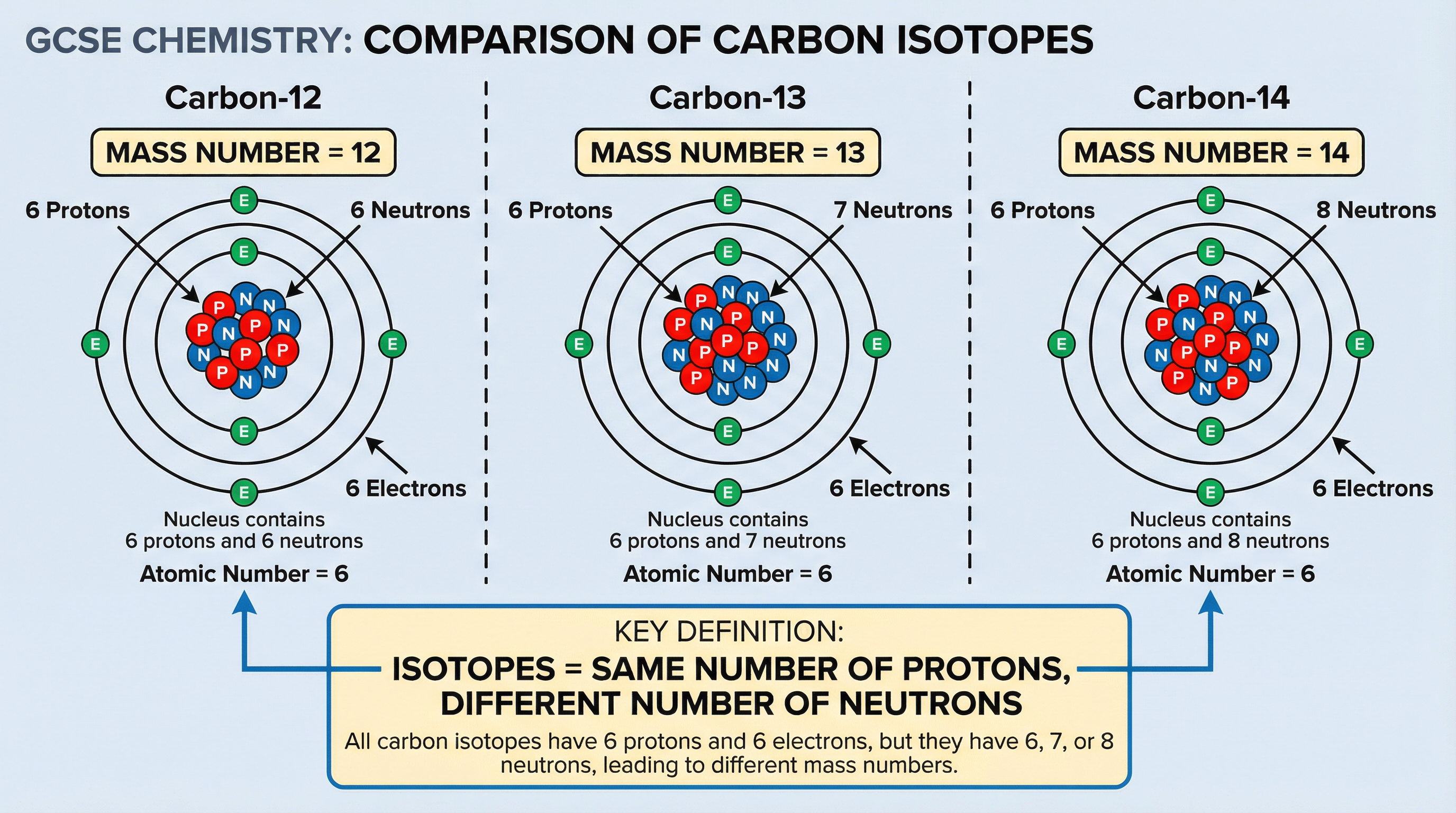
Isotopes are a critical concept. You must be able to define them precisely and use them in calculations.
Definition: Isotopes are atoms of the same element with the same number of protons but a different number of neutrons.
This means isotopes of an element have the same atomic number but different mass numbers. Because they have the same number of electrons, their chemical properties are identical.
Mathematical/Scientific Relationships
Calculating Relative Atomic Mass (Ar)
This is a guaranteed calculation question. The relative atomic mass is the weighted mean mass of an atom of an element compared with one-twelfth of the mass of an atom of carbon-12. You will be given the mass and abundance of isotopes.
Formula (Must memorise):
- If abundances are given as percentages, the total abundance is 100.
- Always show your working, as method marks are awarded.
Example: A sample of bromine contains 50.5% Bromine-79 and 49.5% Bromine-81.
To 1 decimal place, Ar = 80.0
Practical Applications
While there isn't a specific required practical for Atomic Structure itself, the principles are foundational for understanding others. For example, understanding electron shells is vital for explaining reactivity trends in Group 1 and Group 7. The concept of isotopes is crucial in applications like carbon dating (using Carbon-14) and in medical imaging (e.g., Technetium-99m as a tracer).