Study Notes
Overview
Balancing chemical equations is a fundamental skill in chemistry, underpinning all quantitative analysis. It is the practical application of one of the most important laws in science: the Law of Conservation of Mass. In any chemical reaction, atoms are not created or destroyed; they are simply rearranged. A balanced symbol equation is a representation of this, showing the exact same number of atoms of each element on both the reactant (left) and product (right) sides. For your Edexcel GCSE, examiners will test your ability to balance equations in a variety of contexts, from simple synthesis reactions to more complex combustion and displacement reactions. Mastery of this topic is crucial as it forms the foundation for reacting mass calculations, mole concepts, and stoichiometry, which are heavily weighted in the exam.
Key Concepts
Concept 1: The Law of Conservation of Mass
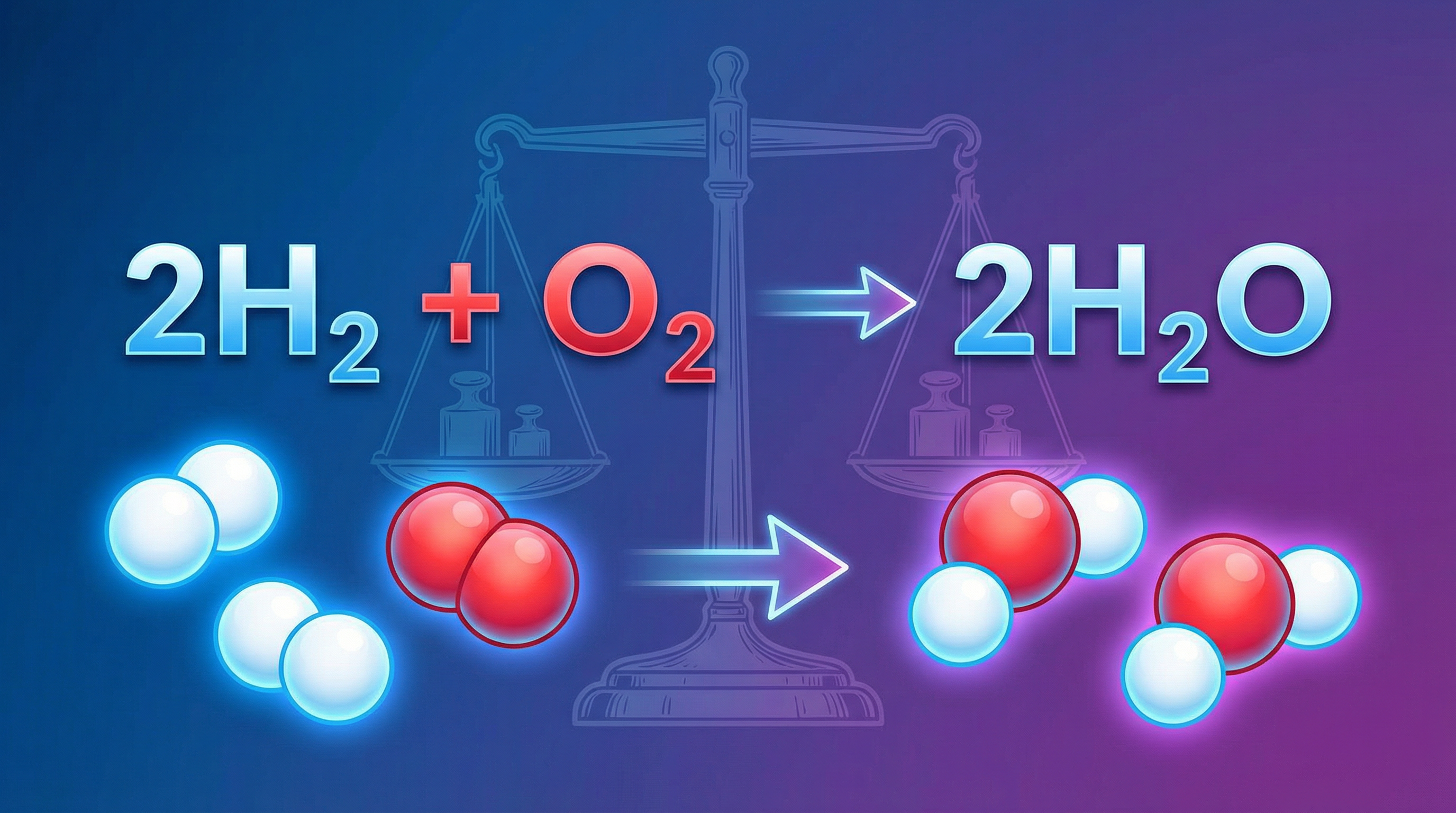
This law states that mass is conserved in a closed system. When you see a chemical equation, you are seeing a model of this law in action. The total mass of all the reactants must equal the total mass of all the products. To achieve this, we must have the same number and type of atoms before and after the reaction arrow. If you start with 2 atoms of hydrogen, you must end with 2 atoms of hydrogen. They might be bonded to different elements, but they are still there.
Example: In the reaction 2H₂ + O₂ → 2H₂O, the mass of two hydrogen molecules plus one oxygen molecule is exactly equal to the mass of two water molecules.
Concept 2: Coefficients vs. Subscripts
This is the most critical distinction to understand.
- Subscripts (the small numbers like the '2' in H₂O) tell you how many atoms are in a single molecule. You CANNOT change these. Altering a subscript changes the chemical identity of the substance (e.g., changing H₂O to H₂O₂ turns water into hydrogen peroxide, a completely different chemical).
- Coefficients (the large numbers in front of a formula, like the '2' in 2H₂O) tell you how many of that entire molecule or formula unit you have. This is the ONLY thing you are allowed to change when balancing an equation.
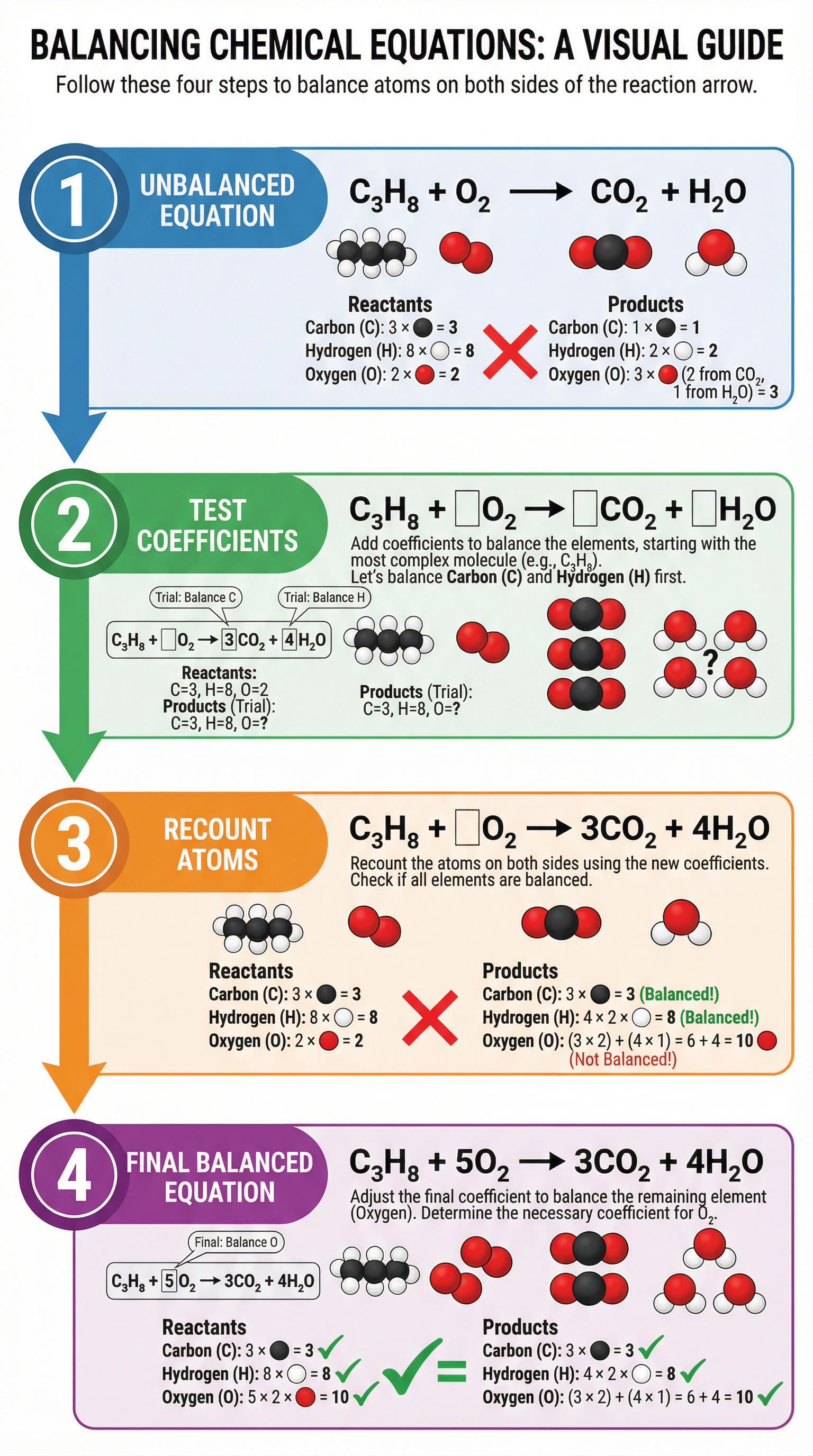
Concept 3: The Systematic Approach (M-N-H-O Rule)
To avoid getting into a loop of changing coefficients back and forth, a systematic approach is essential. A reliable method is to balance elements in the following order:
- Metals
- Non-metals (excluding Hydrogen and Oxygen)
- Hydrogen
- Oxygen
This works because hydrogen and oxygen often appear in multiple reactants and products, so leaving them until last makes the final balancing step much simpler.
Mathematical/Scientific Relationships
The core relationship is simple:
Total atoms of Element X on reactant side = Total atoms of Element X on product sideTo calculate the number of atoms, you use the formula:
Number of atoms = Coefficient × SubscriptFor compounds with brackets, like Ca(NO₃)₂, remember to multiply the subscript outside the bracket by the subscript of each element inside the bracket.
- Nitrogen atoms = 1 (Ca) × 1 (coefficient, implied) × 2 (bracket subscript) = 2
- Oxygen atoms = 1 (Ca) × 1 (coefficient, implied) × 3 (O subscript) × 2 (bracket subscript) = 6
Practical Applications
Balancing equations is not just an academic exercise; it is vital in the real world. For example, in the pharmaceutical industry, chemists must use balanced equations to calculate the exact amounts of reactants needed to produce a specific quantity of a drug, ensuring purity and avoiding waste. In manufacturing, such as the Haber process for making ammonia (N₂ + 3H₂ → 2NH₃), balanced equations are used to optimize the reaction conditions and maximize the yield of the product, which is a key ingredient in fertilizers that help feed the world.
