Study Notes
Overview
Calculating Energy Changes is one of the most exam-focused topics in Edexcel GCSE Chemistry. It requires candidates to apply the principle that breaking chemical bonds requires energy input (endothermic), while forming new bonds releases energy (exothermic). The overall energy change of a reaction is the difference between these two processes. This topic appears regularly in both Foundation and Higher tier papers, often as multi-step calculation questions worth 4-6 marks. Understanding this topic is essential not only for success in the Energetics section but also for synoptic questions that link energy changes to rates of reaction, equilibrium, and real-world applications such as fuel combustion and hand warmers. Examiners test this topic through quantitative bond energy calculations, qualitative analysis of reaction profiles, and explanation questions that require precise scientific language. Typical exam questions include calculating the energy change for a given reaction using bond energy data, drawing and interpreting reaction profile diagrams, and explaining why a reaction is exothermic or endothermic based on bond energy comparisons.
Key Concepts
Concept 1: Bond Breaking is Endothermic
When a chemical reaction occurs, the first step is always to break the bonds in the reactant molecules. Breaking a chemical bond requires energy to be supplied from the surroundings. This is because energy is needed to overcome the attractive forces between the atoms in the bond. This process is described as endothermic, meaning energy is taken in. Think of it like breaking a stick: you need to apply force (energy) to snap it apart. The amount of energy required to break one mole of a particular bond is called the bond energy, and it is measured in kilojoules per mole (kJ/mol). For example, the bond energy of a C-H bond is approximately 412 kJ/mol, meaning 412 kJ of energy is required to break one mole of C-H bonds.
Example: In the combustion of methane (CH₄), the first step is to break four C-H bonds in the methane molecule and two O=O bonds in the oxygen molecules. Each of these bond-breaking steps requires energy input.
Concept 2: Bond Making is Exothermic
Once the bonds in the reactants have been broken, the atoms rearrange and form new bonds in the product molecules. Forming a chemical bond releases energy to the surroundings. This is because energy is released when atoms come together and form stable bonds. This process is described as exothermic, meaning energy is given out. The amount of energy released when one mole of a particular bond is formed is equal to the bond energy of that bond. For example, when one mole of O-H bonds is formed, 463 kJ of energy is released.
Example: In the combustion of methane, after the bonds are broken, new bonds are formed: four O-H bonds in two water molecules and two C=O bonds in one carbon dioxide molecule. Each of these bond-forming steps releases energy.
Concept 3: Calculating the Overall Energy Change
The overall energy change of a reaction is the difference between the total energy required to break all the bonds in the reactants and the total energy released when all the bonds in the products are formed. This is expressed by the formula:
Energy change (ΔH) = Sum of bond energies of bonds broken - Sum of bond energies of bonds madeIf the energy released when making bonds is greater than the energy required to break bonds, the overall energy change is negative, and the reaction is exothermic (releases energy to the surroundings, temperature increases). If the energy required to break bonds is greater than the energy released when making bonds, the overall energy change is positive, and the reaction is endothermic (takes in energy from the surroundings, temperature decreases).
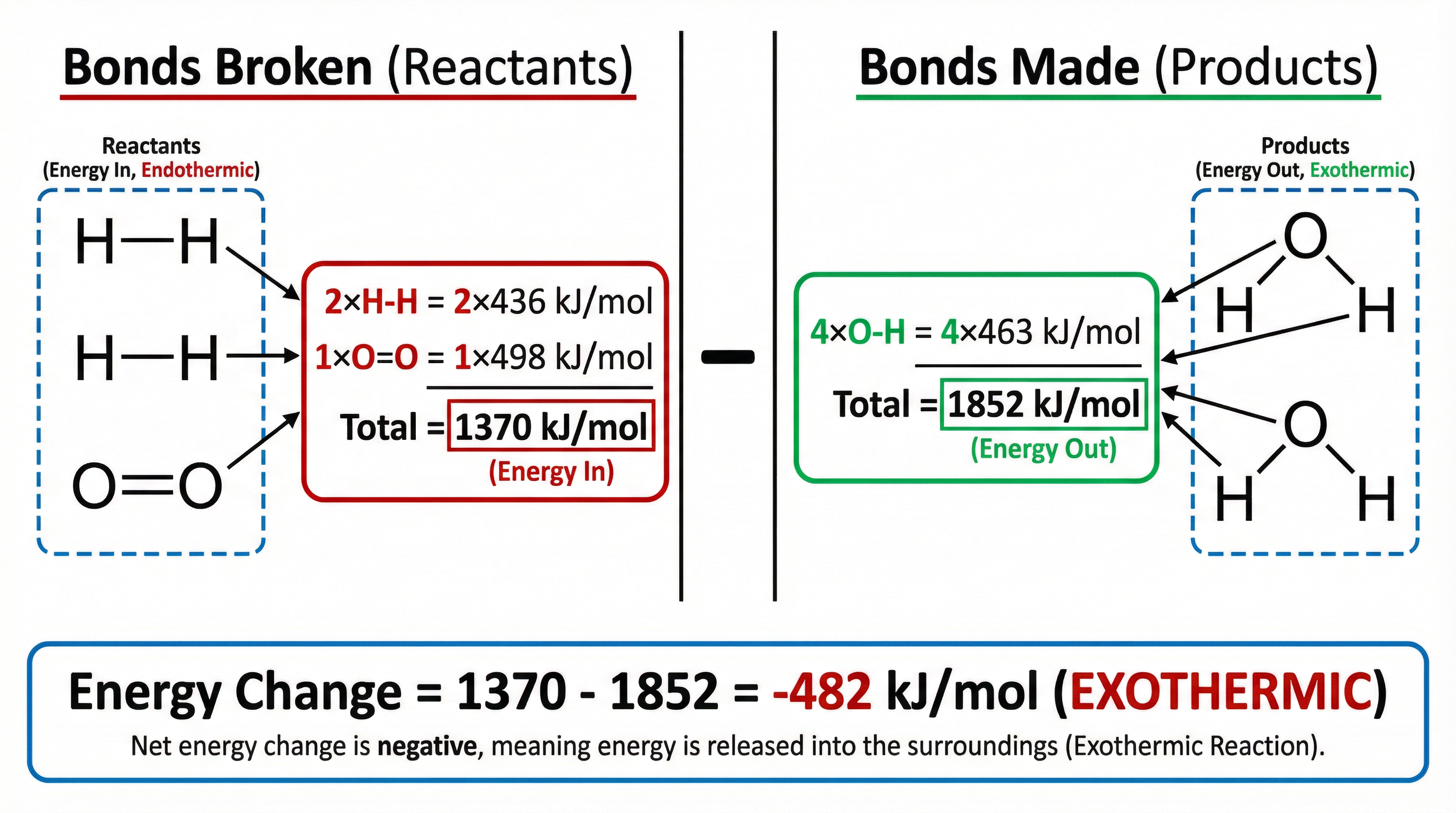
Example: For the reaction 2H₂ + O₂ → 2H₂O:
- Bonds broken: 2 × H-H (2 × 436 kJ/mol) + 1 × O=O (1 × 498 kJ/mol) = 1370 kJ/mol
- Bonds made: 4 × O-H (4 × 463 kJ/mol) = 1852 kJ/mol
- Energy change = 1370 - 1852 = -482 kJ/mol (exothermic)
Concept 4: Reaction Profile Diagrams
A reaction profile diagram is a graphical representation of the energy changes that occur during a chemical reaction. The y-axis represents energy, and the x-axis represents the progress of the reaction. The diagram shows the energy level of the reactants, the energy level of the products, and the activation energy (the minimum energy required to start the reaction). For an exothermic reaction, the products are at a lower energy level than the reactants, and the overall energy change (ΔH) is negative. For an endothermic reaction, the products are at a higher energy level than the reactants, and the overall energy change is positive. It is crucial to distinguish between the activation energy (the energy barrier that must be overcome for the reaction to proceed) and the overall energy change (the difference in energy between reactants and products). Candidates often confuse these two concepts in exam questions.
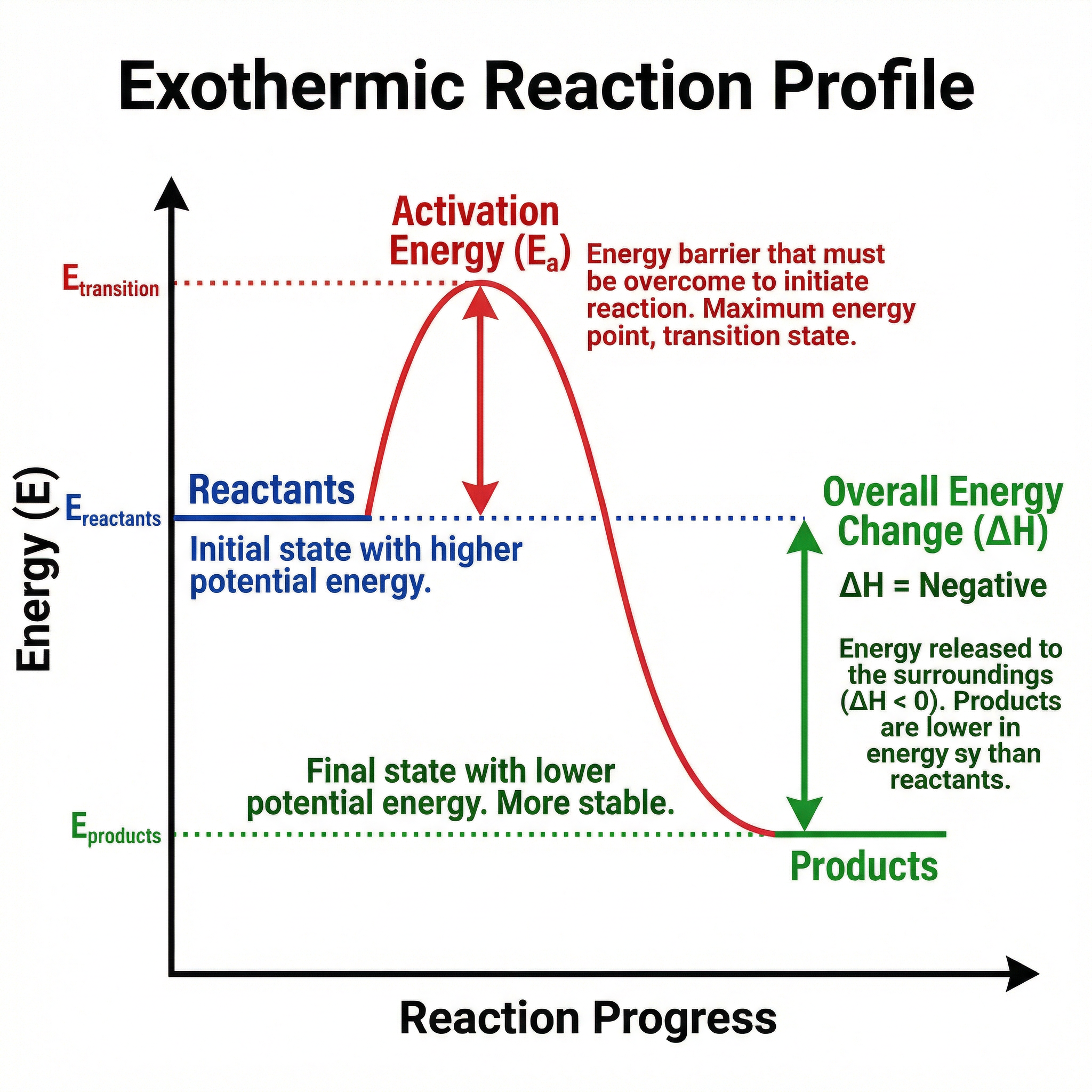
Example: In an exothermic reaction profile, the line starts at the reactants level, rises to a peak (activation energy), and then falls to a lower products level. The difference between the reactants and products levels is the overall energy change, which is negative.
Mathematical/Scientific Relationships
Key Formula (Must Memorise):
Energy change (ΔH) = Σ(Bond energies of bonds broken) - Σ(Bond energies of bonds made)
Where:
- ΔH = Overall energy change of the reaction (kJ/mol)
- Σ(Bond energies of bonds broken) = Total energy required to break all bonds in the reactants (kJ/mol)
- Σ(Bond energies of bonds made) = Total energy released when forming all bonds in the products (kJ/mol)
Sign Convention:
- If ΔH is negative (ΔH < 0), the reaction is exothermic (releases energy)
- If ΔH is positive (ΔH > 0), the reaction is endothermic (absorbs energy)
Important Note: Bond energy values are always given as positive numbers because they represent the energy required to break a bond. When you calculate the energy released when making bonds, you are effectively using the same values but in the context of bond formation.
Practical Applications
Understanding energy changes in chemical reactions has numerous real-world applications. Exothermic reactions are used in hand warmers, self-heating cans, and combustion engines. For example, the combustion of fuels such as methane, petrol, and diesel releases large amounts of energy that can be harnessed for heating and transportation. Endothermic reactions are used in instant cold packs for sports injuries, where a chemical reaction absorbs energy from the surroundings, causing a temperature drop. In industry, understanding energy changes is crucial for optimising reaction conditions, improving energy efficiency, and designing safer processes. For instance, the Haber process for ammonia production is exothermic, and controlling the temperature is essential to maximise yield while managing the energy released.
Podcast: Calculating Energy Changes Explained
Listen to this 10-minute podcast for a comprehensive overview of the topic, including core concepts, exam tips, common mistakes, and a quick-fire recall quiz to test your understanding.
