Study Notes

Overview
Welcome to the essential guide for Metallic Bonding (OCR GCSE Chemistry, Topic 1.8). This topic is fundamental, not just for questions directly about metals, but for higher-level questions that require you to compare and contrast different types of chemical structures. Examiners are looking for precise language and a clear understanding of the 'electron sea' model. You will be expected to describe the giant metallic lattice, composed of positive ions surrounded by delocalised electrons, and use this model to explain physical properties. A common exam question style is the 6-mark extended response, where you must link structure to properties in a logical, well-structured answer. Mastering this topic provides a strong foundation for understanding materials science and electrochemistry, which appear later in the specification.
Key Concepts
Concept 1: The Giant Metallic Lattice
At the heart of this topic is the structure of metals. Unlike simple molecules, metals form a giant metallic lattice. The word 'giant' is crucial as it signifies a vast, repeating three-dimensional arrangement of particles. These particles are not atoms, but positive ions (or cations). This is a point where candidates frequently lose marks. The metal atoms have lost their outer shell electrons, leaving them with a positive charge. These positive ions are packed closely together in regular, orderly layers.
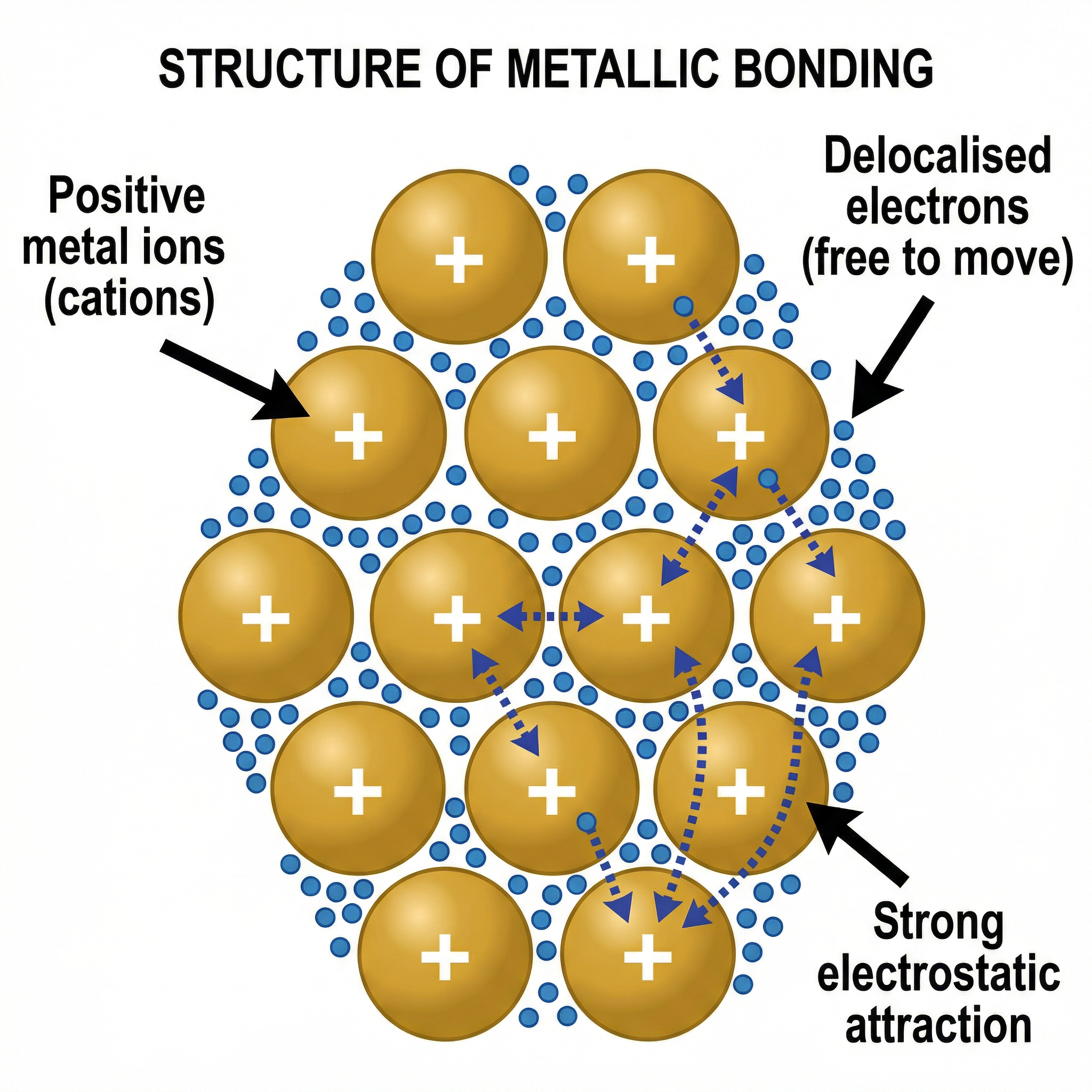
Concept 2: The Sea of Delocalised Electrons
The electrons lost from the outer shells of the metal atoms are not attached to any single ion. Instead, they become delocalised, meaning they are free to move throughout the entire lattice. This creates what is often described as a 'sea of delocalised electrons' surrounding the fixed positive ions. It is vital to use the term 'delocalised' in an exam answer to secure the mark. This mobility of electrons is the key to explaining many of the properties of metals.
Concept 3: The Metallic Bond
The metallic bond itself is the strong electrostatic force of attraction between the positive metal ions and the delocalised sea of electrons. It is this powerful attraction that holds the entire lattice together, giving metals their strength and high melting points. It is incorrect to describe this as an attraction between positive and negative ions (that's ionic bonding) or as sharing of electrons between atoms (that's covalent bonding). The metallic bond is unique.
Linking Structure to Properties
Examiners will almost always ask you to use the model to explain properties. You must explicitly link the features of the structure to the resulting physical property.
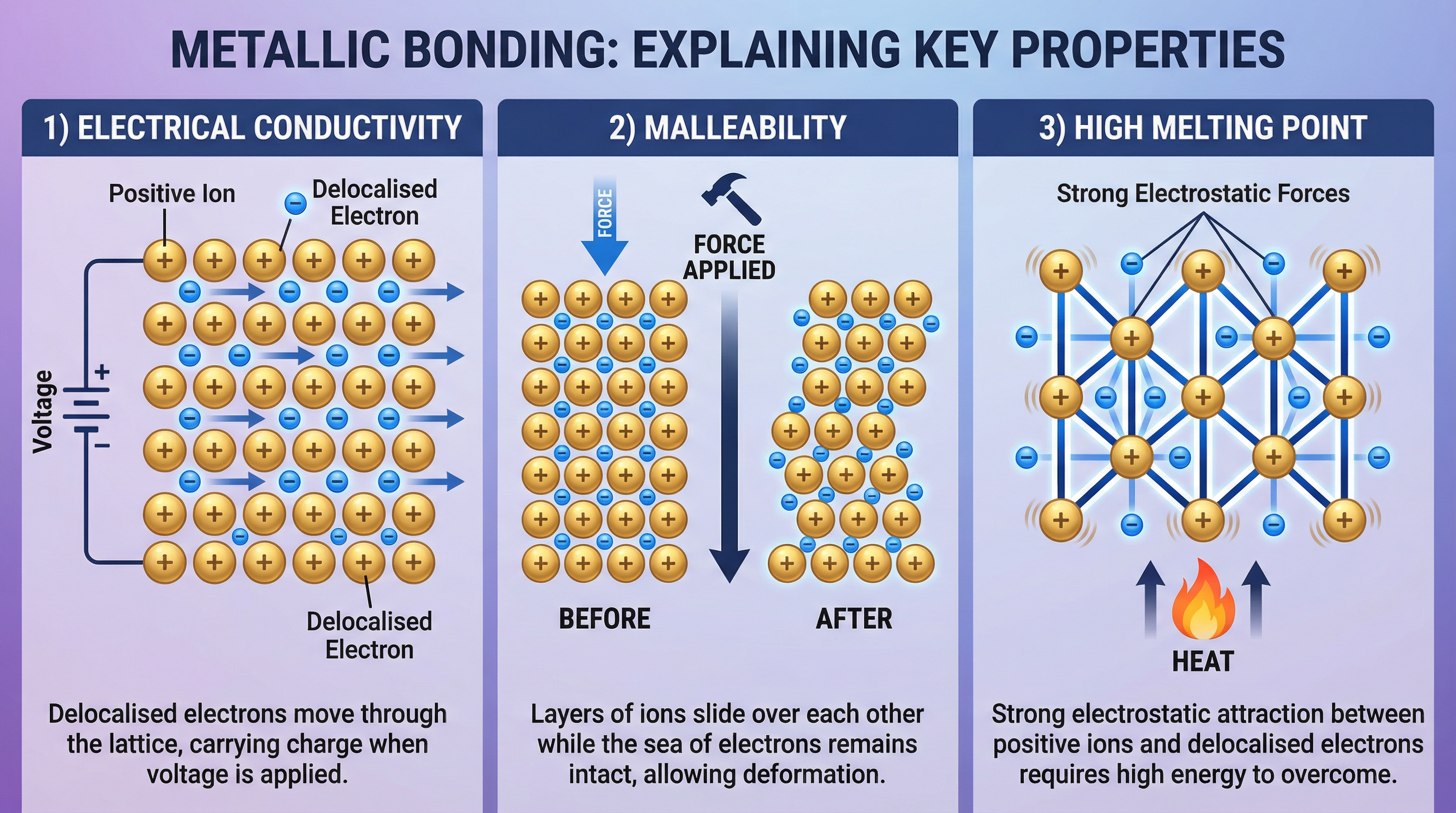
- Electrical Conductivity: Metals are excellent electrical conductors because the delocalised electrons are free to move and carry charge through the lattice when a voltage is applied. The positive ions themselves do not move; they vibrate in fixed positions.
- Malleability and Ductility: Metals can be hammered into shape (malleable) or drawn into wires (ductile). This is because the regular layers of positive ions can slide over one another when a force is applied. The delocalised electrons move with the layers, maintaining the electrostatic attraction, so the metallic bond does not break. The lattice is distorted, not shattered.
- High Melting and Boiling Points: A large amount of thermal energy is required to overcome the strong electrostatic forces of attraction between the positive ions and the delocalised electrons. This is why most metals are solid at room temperature and have high melting points.
- Thermal Conductivity: The delocalised electrons can also transfer kinetic energy quickly throughout the lattice, making metals excellent thermal conductors.
