Study Notes
Overview
Welcome to the core of GCSE Chemistry: Chemical Reactions. This topic is absolutely fundamental, not just for your exams but for understanding the world around you. In this guide, we will dissect the three pillars of WJEC's assessment for topic 3.1: the unshakeable Law of Conservation of Mass and its application in balancing equations; the fascinating world of energy changes in reactions, distinguishing between exothermic and endothermic processes; and for our Higher Tier candidates, the quantitative skill of calculating bond energies to determine the overall enthalpy change of a reaction. A solid grasp of these concepts is essential, as they are frequently tested and form the bedrock for more advanced topics like stoichiometry and reaction rates. Expect to see a variety of question styles, from simple definitions and balancing acts to complex, multi-step calculations and data interpretation. By the end of this guide, you will be equipped to tackle any question the examiner throws at you with confidence and precision.

Key Concepts
Concept 1: The Law of Conservation of Mass & Balancing Equations
The Law of Conservation of Mass is a cornerstone of chemistry. It states that in a closed system, mass is neither created nor destroyed during a chemical reaction. The total mass of the reactants before the reaction is equal to the total mass of the products after the reaction. Atoms are simply rearranged to form new substances. For exam purposes, this means that when you balance a chemical equation, you must have the exact same number of atoms of each element on both sides of the reaction arrow.
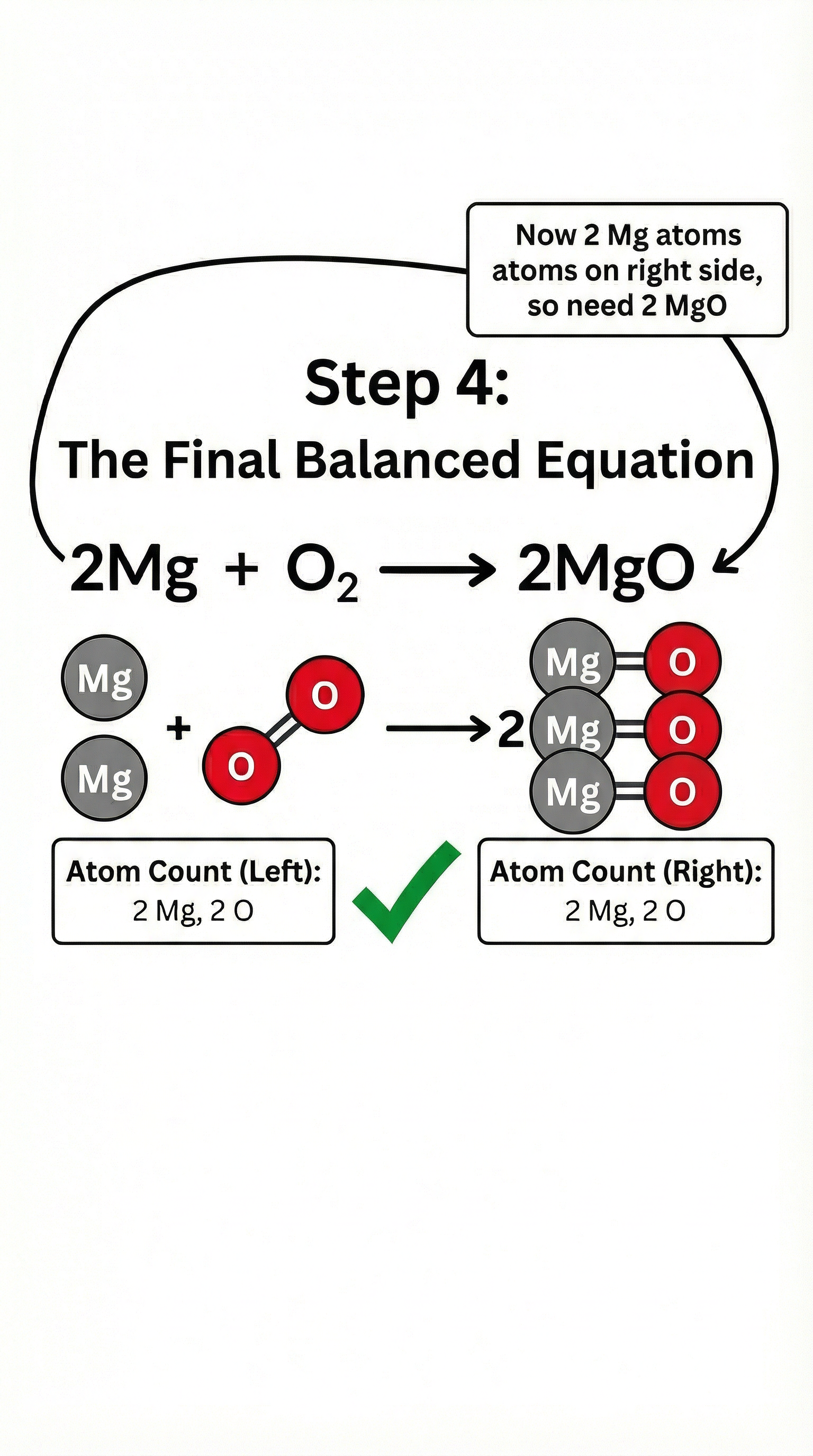
Example: The reaction between methane (CH₄) and oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O).
Unbalanced equation: CH₄ + O₂ → CO₂ + H₂O
Let's count the atoms:
- Reactants side: 1 Carbon, 4 Hydrogen, 2 Oxygen
- Products side: 1 Carbon, 2 Hydrogen, 3 Oxygen
To balance this, we cannot change the chemical formulas (the subscripts). We can only change the coefficients (the large numbers in front of the formulas). Let's balance the hydrogens first. We have 4 on the left and 2 on the right, so we need to place a 2 in front of H₂O.
CH₄ + O₂ → CO₂ + 2H₂O
Now let's recount:
- Reactants side: 1 Carbon, 4 Hydrogen, 2 Oxygen
- Products side: 1 Carbon, 4 Hydrogen, 4 Oxygen
Now the oxygens are unbalanced. We have 2 on the left and 4 on the right. We need to place a 2 in front of O₂.
Balanced equation: CH₄ + 2O₂ → CO₂ + 2H₂O
Let's do a final check:
- Reactants side: 1 Carbon, 4 Hydrogen, 4 Oxygen
- Products side: 1 Carbon, 4 Hydrogen, 4 Oxygen
It's balanced! Credit will be given for correctly balanced symbol equations, and this often carries one or two marks.
Concept 2: Exothermic and Endothermic Reactions
All chemical reactions involve energy changes. This is because chemical bonds are broken and new ones are formed.
- Exothermic reactions release energy into the surroundings, usually in the form of heat. The temperature of the surroundings increases. A classic example is combustion. When you burn a fuel, it feels hot because energy is being given out.
- Endothermic reactions absorb energy from the surroundings. The temperature of the surroundings decreases. A good example is the reaction in a chemical ice pack.
We can represent these energy changes using reaction profiles.
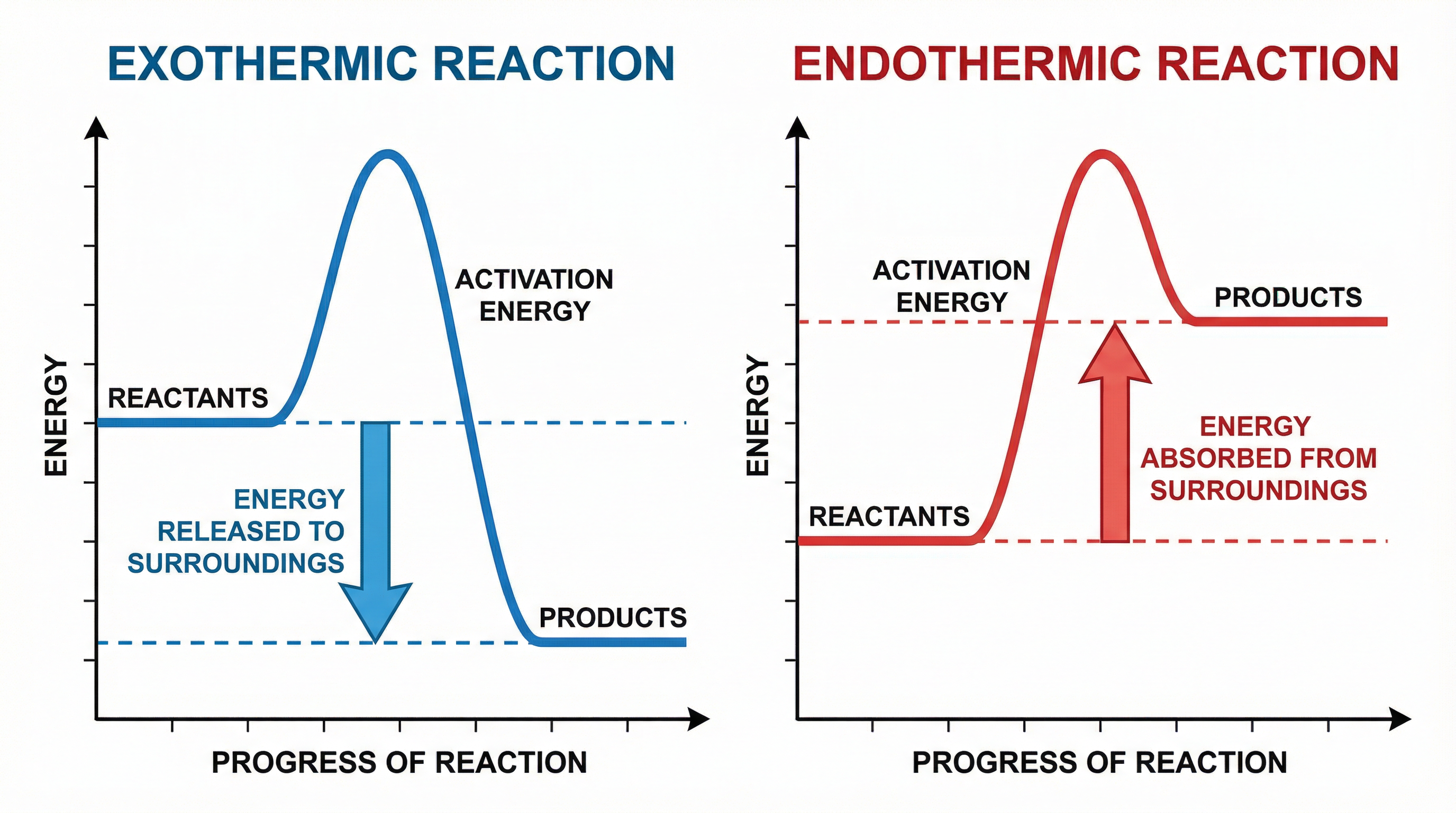
In an exothermic reaction, the products are at a lower energy level than the reactants. The difference in energy is released. In an endothermic reaction, the products are at a higher energy level than the reactants. The difference in energy is absorbed.
Both types of reaction require an initial input of energy to start, known as the activation energy. This is the energy needed to break the existing bonds in the reactants.
Concept 3: Bond Energy Calculations (Higher Tier Only)
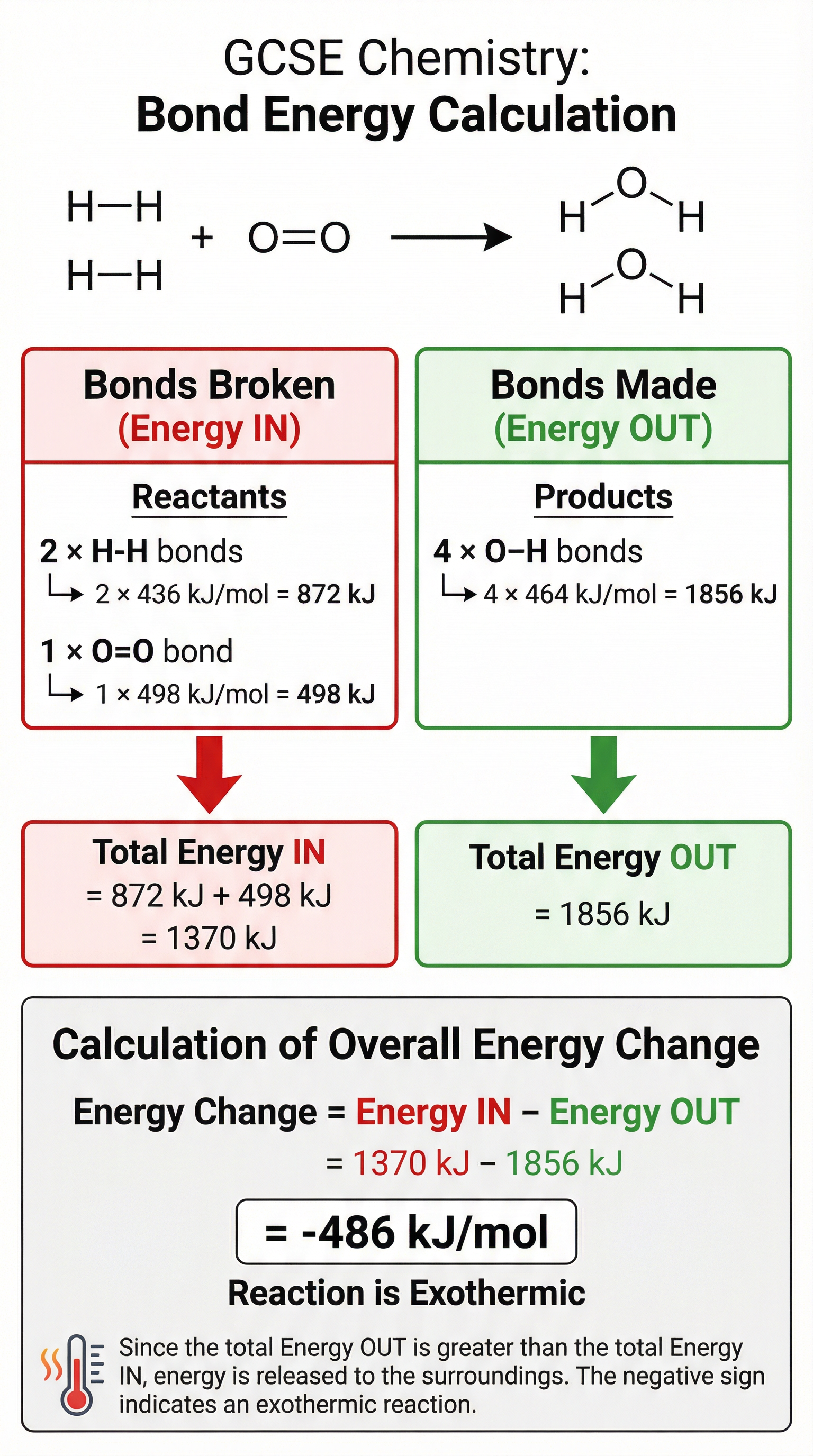
For Higher Tier candidates, you need to be able to calculate the overall energy change of a reaction using bond energies. The key principle is:
- Bond breaking is an endothermic process - it requires energy.
- Bond making is an exothermic process - it releases energy.
The overall energy change (enthalpy change, ΔH) is the difference between the energy required to break bonds and the energy released when new bonds are formed.
**Overall Energy Change = Energy IN (bonds broken) - Energy OUT (bonds made)**If the overall energy change is negative, the reaction is exothermic. If it's positive, the reaction is endothermic.
Mathematical/Scientific Relationships
- Balancing Equations: Ensure that for each element, the number of atoms on the reactant side equals the number of atoms on the product side.
- Bond Energy Calculation (Higher Tier): ΔH = Σ (bond energies of bonds broken) - Σ (bond energies of bonds made)
Practical Applications
- Combustion engines: The combustion of petrol or diesel is a highly exothermic reaction that powers vehicles.
- Self-heating cans and hand warmers: These use exothermic reactions to produce heat on demand.
- Cold packs for sports injuries: These use endothermic reactions to absorb heat and cool the affected area.
Podcast Episode
For a more in-depth discussion of these topics, listen to our podcast episode on Chemical Reactions.
